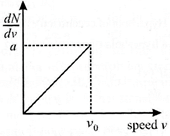
A) The value of \[a{{v}_{0}}\]is\[2N\]
B) The ratio \[v{{~}_{avg}}/{{v}_{0}}\]is equal to \[2/3~\]
C) The ratio \[{{v}_{rms}}/{{v}_{0}}\]is
D) All of the above
Correct Answer: D
Solution :
[d]| Area under the curve is equal to number of molecules of the gas sample. |
| Hence\[N=\frac{1}{2}a{{v}_{0}}\Rightarrow a{{v}_{0}}=2N\] |
| \[{{v}_{avg}}=\frac{1}{N}\int\limits_{0}^{\infty }{v\,N(v)dv=\frac{1}{N}\int\limits_{0}^{{{v}_{0}}}{C\left( \frac{a}{{{v}_{0}}}v \right)dv=\frac{2}{3}{{v}_{0}}}}\] \[\Rightarrow \frac{{{v}_{avg}}}{{{v}_{0}}}=\frac{2}{3}\] |
| \[{{v}_{rms}}=\frac{1}{N}\int\limits_{0}^{\infty }{{{v}^{2}}\,N\left( v \right)dv=\frac{1}{N}\int\limits_{0}^{{{v}_{0}}}{{{v}^{2}}\left( \frac{a}{{{v}_{0}}}v \right)dv=\frac{v_{0}^{2}}{2}}}\] \[\Rightarrow \frac{{{v}_{rms}}}{{{v}_{0}}}=\frac{1}{\sqrt{2}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
