A) \[{{p}^{3/2}}\]
B) \[{{p}^{2/3}}\]
C) \[{{p}^{3}}\]
D) \[{{p}^{2}}\]
Correct Answer: B
Solution :
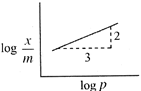
\[\frac{x}{m}=K{{P}^{\text{1/n}}}\] \[\log \frac{x}{m}=\frac{1}{n}\log \rho +\log K\]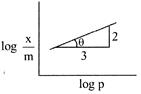 Slope \[=\tan \theta =\frac{2}{3}=\frac{1}{n}\] \[\frac{x}{m}\propto {{p}^{2/3}}.\]
Slope \[=\tan \theta =\frac{2}{3}=\frac{1}{n}\] \[\frac{x}{m}\propto {{p}^{2/3}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
