A) \[0.242\times {{10}^{-4}}at{{m}^{2}}\]
B) \[1\times {{10}^{-4}}at{{m}^{2}}\]
C) \[4.9\times {{10}^{-3}}at{{m}^{2}}\]
D) \[0.242\text{ }at{{m}^{2}}\]
Correct Answer: A
Solution :
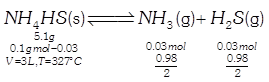 \[{{K}_{P}}={{P}_{N{{H}_{3}}}}{{P}_{{{H}_{2}}S}}\] \[PV=nRT\] \[P\times 3=0.06\times 0.0821\times 600\] \[{{K}_{p}}=\frac{0.98}{2}\times \frac{0.98}{2}\] \[P=\frac{0.06\times 0.0832\times 200}{3}\] \[{{K}_{p}}=0.243\] \[P=0.98\]
\[{{K}_{P}}={{P}_{N{{H}_{3}}}}{{P}_{{{H}_{2}}S}}\] \[PV=nRT\] \[P\times 3=0.06\times 0.0821\times 600\] \[{{K}_{p}}=\frac{0.98}{2}\times \frac{0.98}{2}\] \[P=\frac{0.06\times 0.0832\times 200}{3}\] \[{{K}_{p}}=0.243\] \[P=0.98\]
You need to login to perform this action.
You will be redirected in
3 sec
