A) \[3d_{xy}^{1},3d_{yz}^{1},3d_{xz}^{1}\]
B) \[3d_{xy}^{1},3d_{yz}^{1},3d_{{{z}^{2}}}^{1}\]
C) \[(3d_{{{x}^{2}}-{{y}^{2}}}^{1}),3d_{{{z}^{2}}}^{1},3d_{xz}^{1}\]
D) \[3d_{xy}^{1},(3d_{{{x}^{2}}-{{y}^{2}}}^{1}),3d_{yz}^{1}\]
Correct Answer: A
Solution :
\[C{{r}^{3+}}:1{{s}^{2}}2{{p}^{2}}2{{p}^{6}},3{{s}^{2}}s{{p}^{6}}3{{d}^{3}}.\]The \[3d_{xy}^{1},3d_{zx}^{1},3d_{yz}^{1}\]has lower energy. \[C{{r}^{3+}}\]is \[3{{d}^{3}}\]in presence of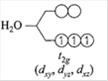
You need to login to perform this action.
You will be redirected in
3 sec
