A) \[\frac{3}{4{{\rho }_{0}}}\]
B) \[\frac{3}{2{{\rho }_{0}}}\]
C) \[\frac{4}{3{{\rho }_{0}}}\]
D) \[2{{\rho }_{0}}\]
Correct Answer: B
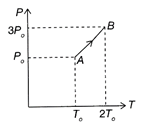
Solution :
\[\rho =\frac{PM}{RT}\]or \[\rho \propto \frac{P}{T}\]or \[{{\left( \frac{P}{T} \right)}_{A}}=\frac{{{P}_{0}}}{{{T}_{o}}}\]and \[{{\left( \frac{P}{T} \right)}_{B}}=\frac{3}{2}\frac{{{P}_{0}}}{{{T}_{o}}}\] \[\therefore \]\[{{\left( \frac{P}{T} \right)}_{B}}=\frac{3}{2}{{\left( \frac{P}{T} \right)}_{A}}\Rightarrow {{\rho }_{B}}=\frac{3}{2}{{\rho }_{A}}=\frac{3}{2}{{\rho }_{0}}\] Hence, the correction option is .You need to login to perform this action.
You will be redirected in
3 sec
