A) linear
B) triangular planar
C) pyramidal
D) square planar
Correct Answer: D
Solution :
\[Xe{{F}_{4}}\] has \[s{{p}^{3}}{{d}^{2}}\] hybridisation. The shape of \[Xe{{F}_{4}}\]molecule is square planar. It has two lone pairs.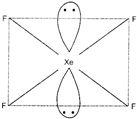
You need to login to perform this action.
You will be redirected in
3 sec
