| (i) Using de-Broglie's hypothesis, explain with the help of a suitable diagram, Bohr's second postulate of quantisation of energy levels in a hydrogen atom. |
| (ii) The ground state energy of hydrogen atom is \[-13.6\text{ }eV\] What are the kinetic and potential energies of the electron in this state? |
Answer:
Bohr?s second postulate (i) According to de-Broglie, a stationary orbit is that which contains an integral number of de-Broglie standing waves associated with the revolving electron. For an electron revolving in nth circular orbit of radius \[{{r}_{n}}\] total distance covered = circumference of the orbit \[=2\pi {{r}_{n}}.\] \[\therefore \] For the permissible orbit, \[2\pi {{r}_{n}}=n\lambda \] According to de-Broglie wavelength, \[\lambda =h/m{{v}_{n}}\] where, \[{{v}_{n}}\] is speed of electron revolving in nth orbit. \[\therefore \] \[2\pi {{r}_{n}}=\frac{nh}{m{{v}_{n}}}\] Or \[m{{v}_{n}}{{r}_{n}}=\frac{nh}{2\pi }=n\,\,(h/2\pi )\] 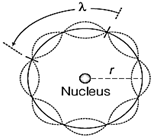 A standing wave is shown on a circular orbit i.e. Angular momentum of electron revolving in nth orbit must be an integral multiple of \[\frac{h}{2\pi },\]which is the quantum condition proposed by Bohr in his second postulate. (ii) \[\therefore \] Total energy of an electron in an atom, \[E=-KE\] and potential energy of an electron in an atom\[,U=2E\] \[\therefore \] \[E=-13.6\,eV\] And \[U=2\times (-13.6\,eV)=-\,27.2\,eV\]
A standing wave is shown on a circular orbit i.e. Angular momentum of electron revolving in nth orbit must be an integral multiple of \[\frac{h}{2\pi },\]which is the quantum condition proposed by Bohr in his second postulate. (ii) \[\therefore \] Total energy of an electron in an atom, \[E=-KE\] and potential energy of an electron in an atom\[,U=2E\] \[\therefore \] \[E=-13.6\,eV\] And \[U=2\times (-13.6\,eV)=-\,27.2\,eV\]
You need to login to perform this action.
You will be redirected in
3 sec
