Answer:
Example To separate acetone and water from their mixture.
Procedure
(i) Take the mixture in a distillation flask. Fit it with a thermometer.
(ii) Set the apparatus as shown in the figure given below.
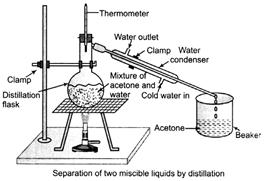 (iii) Heat the mixture slowly and note the reading of thermometer.
(iv) The acetone vaporizes condenser in condenser an can be collected in the beaker.
(v) Water is left behind in the distillation flask. (2)
OR
Difference between true solutions, suspension and colloid
(iii) Heat the mixture slowly and note the reading of thermometer.
(iv) The acetone vaporizes condenser in condenser an can be collected in the beaker.
(v) Water is left behind in the distillation flask. (2)
OR
Difference between true solutions, suspension and colloid
Characteristics
True solution
Suspension
Colloid
Type of mixture
Homogeneous
Heterogeneous
Heterogeneous but appears to be homogeneous.
Stability
Stable, particles do not settle down on keeping.
Not stable, particulars settle down filter paper.
Stable, particles do not settle down on keeping.
Filterability
Passes through filter paper.
Do not pass through filter paper.
Passes through filter paper.
Size of solute
Very small i.e., less than
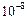 cm in diameter.
cm in diameter.
Quite large i.e., large than
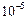 cm in diameter
cm in diameter
Between
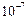 cm and
cm and 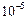 cm in diameter
cm in diameter
Visibility of particles
Not visible even with a powerful microscope.
Visible even with naked eyes.
Visible with the help of a microscope

You need to login to perform this action.
You will be redirected in
3 sec
