Answer:
Metal nitrate A is 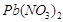 .
(I)
.
(I) 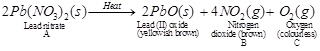 (II)
(II) 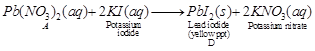 (3)
A is lead nitrate, B is nitrogen dioxide, C is oxygen and D is lead iodide. (1)
Reaction I is decomposition reaction and II is double displacement reaction (precipitation reaction). (1)
Or
(a)
(3)
A is lead nitrate, B is nitrogen dioxide, C is oxygen and D is lead iodide. (1)
Reaction I is decomposition reaction and II is double displacement reaction (precipitation reaction). (1)
Or
(a)  is acid and NaOH is base whose combination forms the common salt. (1)
Its formula is NaCl (Sodium chloride).
It is obtained from sea water.
is acid and NaOH is base whose combination forms the common salt. (1)
Its formula is NaCl (Sodium chloride).
It is obtained from sea water.  (b) Rock salt is the common name for the mineral "halite". Its chemical formula is NaCl. (1)
It may be white or light blue or yellow depending upon impurities present in it.
(b) Rock salt is the common name for the mineral "halite". Its chemical formula is NaCl. (1)
It may be white or light blue or yellow depending upon impurities present in it. 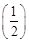 (c)
(c) 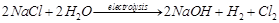
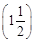
You need to login to perform this action.
You will be redirected in
3 sec
