Answer:
(i)![]() Smaller the value of
Smaller the value of ![]() ,
larger the value of
,
larger the value of![]() .
.
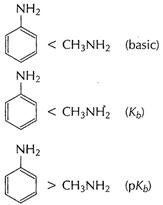
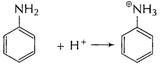
 Basic character of
Basic character of ![]() is due
to lone pair of N-atom. In aniline (A) electron-density at N-atom is decreased as
this lone pair is used in delocalisation of Ti-electrons in benzene nucleus.
Thus,
is due
to lone pair of N-atom. In aniline (A) electron-density at N-atom is decreased as
this lone pair is used in delocalisation of Ti-electrons in benzene nucleus.
Thus, ![]() is less
basic than
is less
basic than![]()

![]() group
is electron donating group. This increases electron density at N-atoms. Thus,
group
is electron donating group. This increases electron density at N-atoms. Thus, ![]() is more basic
than
is more basic
than ![]() , Hence
, Hence
![]() Thus,
Thus,
 [1]
(ii) Nitration is done using HN03 (after protecting
[1]
(ii) Nitration is done using HN03 (after protecting ![]() group
by acetylation)
group
by acetylation) ![]() group
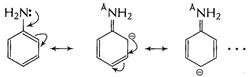
increases electron-density at o- and p-sites as shown
group
increases electron-density at o- and p-sites as shown
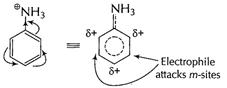
 Due to presence of
Due to presence of ![]() group
is acidified
group
is acidified
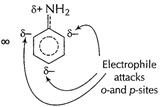
 and thus
and thus![]() group is
now, electron-withdrawing making benzene nucleus electron-deficient at o- and p-sites
and only m-sites available but with decreased reactivity.
group is
now, electron-withdrawing making benzene nucleus electron-deficient at o- and p-sites
and only m-sites available but with decreased reactivity.
You need to login to perform this action.
You will be redirected in
3 sec
