Answer:
(a) (i) ![]() is a
better reducing agent than
is a
better reducing agent than ![]()
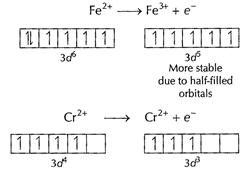 In the formation of
In the formation of ![]() stability
increases hence
stability
increases hence![]() is
a better reducing agent. [1]
(ii)
is
a better reducing agent. [1]
(ii)![]()
![]() Since E° is positive it indicates that
Since E° is positive it indicates that ![]() is a
better oxidising agent. It can oxidise
is a
better oxidising agent. It can oxidise ![]() but
rate is very slow hence it is a good analytical reagent. [1]
(iii)In aqueous solution, C^ undergoes disproportions
but
rate is very slow hence it is a good analytical reagent. [1]
(iii)In aqueous solution, C^ undergoes disproportions
![]()
![]() Thus, it
is spontaneous.
Thus, it
is spontaneous.
![]() is
thus unstable in aqueous solution. [1]
(b)
is
thus unstable in aqueous solution. [1]
(b)![]() [1]
[1]
![]()
![]() [1]
[1]
You need to login to perform this action.
You will be redirected in
3 sec
