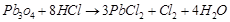 (ii)
(ii) 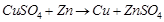 (iii)
(iii) 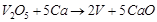 Or
(a) Crystals of a substance changed their colour on heating in a closed test tube but regained it after sometime when they were allowed to cool down. Name the substance and write its formula and explain the phenomenon involved.
(b) Name the compound whose one formula unit is associated with 10 water molecules. How is it prepared? Give equations of related reactions. Give two uses of the compound.
Or
(a) Crystals of a substance changed their colour on heating in a closed test tube but regained it after sometime when they were allowed to cool down. Name the substance and write its formula and explain the phenomenon involved.
(b) Name the compound whose one formula unit is associated with 10 water molecules. How is it prepared? Give equations of related reactions. Give two uses of the compound.
Answer:
(a) Fireflies glow at night because protein present in fireflies undergoes oxidation in the presence of air and an enzyme. This chemical reaction involves emission of visible light. This shows that fireflies glow at night. (2)
(b) (i) 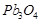 (Red lead) is an oxidising agent.
(ii)
(Red lead) is an oxidising agent.
(ii) 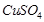 is an oxidising agent.
(iii)
is an oxidising agent.
(iii) 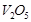 is an oxidising agent. (1 x 3)
Or
(a)
is an oxidising agent. (1 x 3)
Or
(a) 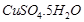 is blue crystalline solid. It becomes dirty white on heating due to loss of water molecules and it becomes amorphous.
is blue crystalline solid. It becomes dirty white on heating due to loss of water molecules and it becomes amorphous.
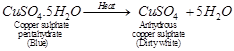 It regains its colour by absorbing water from atmosphere and becomes blue in colour.
It regains its colour by absorbing water from atmosphere and becomes blue in colour.
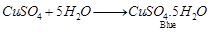 (2)
(b)
(2)
(b) 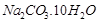 . It is called sodium carbonate decahydrate or washing soda. It is prepared by passing
. It is called sodium carbonate decahydrate or washing soda. It is prepared by passing 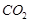 gas through saturated solution of ammonical brine.
gas through saturated solution of ammonical brine.
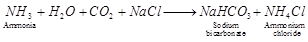
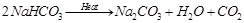
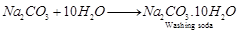 (2)
Uses
(i) It is used in production of washing powder.
(ii) It is used for manufacture of glass. (1/2x2)
(2)
Uses
(i) It is used in production of washing powder.
(ii) It is used for manufacture of glass. (1/2x2)
You need to login to perform this action.
You will be redirected in
3 sec
