Answer:
When
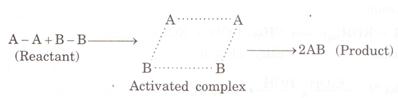
reactants changes into products, they have to cross the energy barrier. ![]() When the reactant absorbs energy, their old bonds are
loosened and new bonds start forming between them. This highly unstable
transition state between reactants and products is called activated complex. 1
When the reactant absorbs energy, their old bonds are
loosened and new bonds start forming between them. This highly unstable
transition state between reactants and products is called activated complex. 1
 Activation energy = Energy of activated complex - Average
energy of reactants
Activation energy = Energy of activated complex - Average
energy of reactants ![]()
You need to login to perform this action.
You will be redirected in
3 sec
