A) Absolutely pure water does not contain any ions
B) London forces is directly proportional to more number of loosely held electron
C) Allene is polar due to its non-planar structure
D) \[C{{H}_{3}}OC{{H}_{3}}\] is less volatile than \[{{C}_{2}}{{H}_{5}}OH\] due to higher molecular weight.
Correct Answer: B
Solution :
[b]| (1) \[{{H}_{2}}O\rightleftharpoons {{H}^{+}}+O{{H}^{-}}\] |
| \[{{K}_{w}}={{10}^{-14}}\] at \[{{25}^{o}}\] |
| Even in conductivity water \[({{H}_{2}}O)\] is found to be dissociated into \[{{H}^{+}}\] and \[O{{H}^{-}}\]. |
| (2) More the number of loosely held electron, greater will be their tendency for being polarized deformed i.e., the magnitude of formal charge increases, hence London forces increases. |
| (3) \[{{H}_{2}}C=C=C{{H}_{2}}\] |
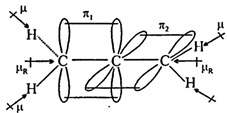 |
| (Non planar) \[{{\mu }_{D}}=0\] |
| (4) In \[C{{H}_{3}}COC{{H}_{3}}\] there is no H-bonding hence more volatile, while in \[{{C}_{2}}{{H}_{5}}OH\] intermolecular H-bonding is present hence less volatile, due to association. |
You need to login to perform this action.
You will be redirected in
3 sec
