| A decomposes as |
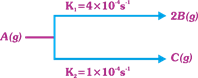 |
| The rate of disappearance of B, taking 4M concentration of A is equal to |
A) \[1.6\times {{10}^{-3}}M{{s}^{-1}}\]
B) \[6.4\times {{10}^{-3}}M{{s}^{-1}}\]
C) \[3.2\times {{10}^{-3}}M{{s}^{-1}}\]
D) insufficient to calculate
Correct Answer: C
Solution :
\[\frac{1}{2}\frac{d{{C}_{B}}}{dt}={{K}_{1}}{{C}_{A}}\] \[\frac{d{{C}_{B}}}{2dt}=2{{K}_{1}}{{C}_{A}}\] \[=2\times 4\times {{10}^{-4}}\times 4=32\times {{10}^{-4}}\] \[=3.2\times {{10}^{-3}}\text{M}\,{{\text{s}}^{-1}}\] (logical)You need to login to perform this action.
You will be redirected in
3 sec
