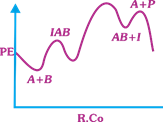
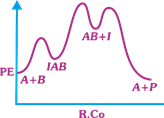
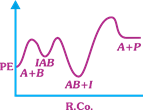
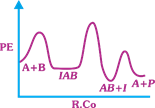
| The following mechanism has been proposed for the exothermic catalyzed complex reaction |
| \[A+BIAB\xrightarrow[{}]{{{k}_{1}}}AB+I\xrightarrow[{}]{{{k}_{2}}}Q+A\] |
| If \[{{k}_{1}}\] is much smaller than \[{{k}_{2}},\] the most suitable qualitative plot of Potential Energy (PE) versus reaction coordinate (R.Co) for the above reaction. |
A)
B)
C)
D)
Correct Answer: B
Solution :
| \[A+BlAB\] so \[{{E}_{a(f)}}\] its high and \[{{E}_{a(b)}}\] is low \[({{k}_{1}}<<{{k}_{2}});{{E}_{a}}\] for this step is very high and next step is low and overall reaction is exothermic. |
You need to login to perform this action.
You will be redirected in
3 sec
