A)
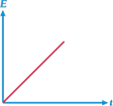
B)

C)

D)
Correct Answer: C
Solution :
| [c] Mean KE of gas molecule \[E=\frac{3}{2}kT=\frac{3}{2}k(t+273)\] |
| where \[T=\] temperature is in kelvin and \[t=\] is in centigrade. |
| \[\therefore \] \[\,E=\frac{3}{2}kt+\frac{3}{2}\,\times 273\,k\] |
| [k = Boltzmann's constant] |
| By comparing this equation with standard equation of straight line |
| \[y=mx+c,\] we get \[m=\frac{3}{2}k\] and \[c=\frac{3}{2}\,273\,k\]. |
| So, the graph between E and t will be straight line with positive intercept on y-axis and positive slope with r-axis. |
You need to login to perform this action.
You will be redirected in
3 sec
