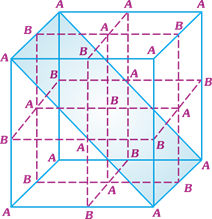
A) \[AB\]
B) \[{{A}_{5}}{{B}_{7}}\]
C) \[{{A}_{7}}{{B}_{5}}\]
D) none of these
Correct Answer: A
Solution :
| [a] Effective number of (particles removed |
| \[=4\times \frac{1}{8}+2\times \frac{1}{2}=\frac{3}{2}\] |
| Effective number of B particles removed |
| \[=2\times \frac{1}{4}+1=\frac{3}{2}\] |
| Effective number of B particles present in a unit cell \[=4-\frac{3}{2}=\frac{5}{2}\] |
| A : B |
| \[\frac{5}{2}\] : \[\frac{5}{2}\] or \[1:1\] |
You need to login to perform this action.
You will be redirected in
3 sec
