| DIRECTION: Read the passage given below and answer the questions that follows: |
| In the figure n mole of a monoatomic ideal gas undergo the process ABC as shown in the P-V diagram. The process AB is isothermal and BC is isochoric. The temperature of the gas at A is \[{{T}_{0}}\]. Total heat given to the gas during the process ABC is measured to be Q. |
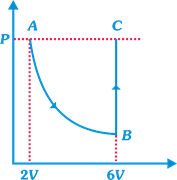 |
| The average molar heat capacity of the gas in process ABC |
A) \[\frac{Q}{n{{T}_{0}}}\]
B) \[\frac{Q}{2n{{T}_{0}}}\]
C) \[\frac{Q}{3n{{T}_{0}}}\]
D) \[\frac{2Q}{n{{T}_{0}}}\]
Correct Answer: B
Solution :
[b]Heat capacity is given by \[C=\frac{1}{n}\frac{dQ}{dT};C=\frac{1}{n}\frac{Q}{2{{T}_{0}}}\]You need to login to perform this action.
You will be redirected in
3 sec
