| A certain mass of gas is taken from an initial thermodynamic state A to another state B by process I and II. In process I the gas does 5 joules of work and absorbs 4 joules of heat energy. In process II, the gas absorbs 5 joules of heat. The work done by the gas in process II (see figure) is |
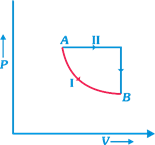 |
A) +6 joules
B) - 6 joules
C) +4 joules
D) - 4 joules
Correct Answer: A
Solution :
| [a]\[\Delta H=\Delta U+\Delta W\] |
| \[\Delta {{U}_{1}}=\Delta {{U}_{2}}\] |
| \[{{(\Delta H-\Delta W)}_{1}}=(\Delta H-\Delta W)\] |
| \[4-5=5-\Delta W\] |
| \[\frac{{{x}^{2}}}{{{a}^{2}}}+\frac{{{y}^{2}}}{{{b}^{2}}}=1\] |
You need to login to perform this action.
You will be redirected in
3 sec
