A)
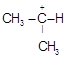
B)
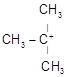
C)
![]()
D)
![]()
Correct Answer: B
Solution :
| [b] The most stable carbocation is t-alkyl carbocation because the order of stability of alkyl carbocation is t-alkyl> s-alkyl > p-alkyl > |
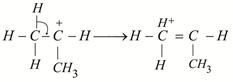 |
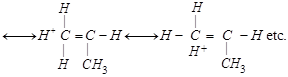 |
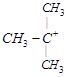 shows nine resonating structures shows nine resonating structures |
| due to presence of nine |
| |
You need to login to perform this action.
You will be redirected in
3 sec
