A) V
B) Cr
C) Mn
D) Fe
Correct Answer: B
Solution :
Write the electronic configuration of all the given metals according to Hund's and Pauli exclusion rules. Find the stability of metal after ejection of one electron.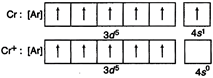 (by first IP) This is stable EC hence formation of \[C{{r}^{2+}}\] by second IP requires maximum enthalpy.
(by first IP) This is stable EC hence formation of \[C{{r}^{2+}}\] by second IP requires maximum enthalpy.
You need to login to perform this action.
You will be redirected in
3 sec
