A) \[S{{F}_{4}}\]
B) \[Si{{F}_{4}}\]
C) \[Xe{{F}_{4}}\]
D) \[B{{F}_{4}}\]
Correct Answer: A
Solution :
\[S{{F}_{4}}\]has trigonal bipyramidal geometry, lone pair of electrons repels the axial bond pair and decreases the bond angle to\[173{}^\circ \].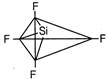 \[Si{{F}_{4}}\]Regular tetrahedral
\[Si{{F}_{4}}\]Regular tetrahedral 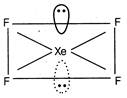 \[Xe{{F}_{4}}\] Square planar
\[Xe{{F}_{4}}\] Square planar 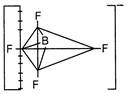 \[BF_{4}^{-}\]Tetrahedral
\[BF_{4}^{-}\]Tetrahedral
You need to login to perform this action.
You will be redirected in
3 sec
