question_answer 1) The path difference for destructive interference is:
A)
\[{{T}^{2}}=k{{R}^{3}}\]
done
clear
B)
\[\frac{{{T}_{2}}}{{{T}_{1}}}={{\left( \frac{{{R}_{2}}}{{{R}_{1}}} \right)}^{3/2}}={{\left( \frac{1.01R}{R} \right)}^{3/2}}\]
done
clear
C)
\[\Rightarrow \]
done
clear
D)
\[\frac{{{T}_{2}}}{{{T}_{1}}}={{(1+0.01)}^{3/2}}=1+\frac{3}{2}\times 0.01\]
done
clear
View Answer play_arrow
question_answer 2) A black body is at a temperature 300 K. It emits energy at a rate, which is proportional to :
A)
\[{{(300)}^{4}}\]
done
clear
B)
\[{{(300)}^{3}}\]
done
clear
C)
\[{{(300)}^{2}}\]
done
clear
D)
300
done
clear
View Answer play_arrow
question_answer 3) The density of a substance at 0°C is 10 g/cc and at 100°C, its density is 9.7 g/cc. The coefficient of linear expansion of the substance is:
A)
\[{{10}^{-4}}\]
done
clear
B)
\[{{10}^{-2}}\]
done
clear
C)
\[{{10}^{-3}}\]
done
clear
D)
\[{{10}^{2}}\]
done
clear
View Answer play_arrow
question_answer 4) The kinetic energy of a body becomes four times its initial value. The new linear momentum will be:
A)
eight times that of initial value
done
clear
B)
four times that of initial value
done
clear
C)
twice of the initial value
done
clear
D)
remain as the initial value
done
clear
View Answer play_arrow
question_answer 5) The dimension of torque is:
A)
\[\frac{\Delta T}{T}\times 100=\left( \frac{{{T}_{2}}}{{{T}_{1}}}-1 \right)\times 100\]
done
clear
B)
\[=\frac{3}{2}\times 0.01\times 100\]
done
clear
C)
\[=1.5%\]
done
clear
D)
\[Y=3K(1-2\sigma )\]
done
clear
View Answer play_arrow
question_answer 6) A 0 K temperature, a p-type semiconductor:
A)
has equal numbers of holes and free electrons
done
clear
B)
has few holes but no free electrons
done
clear
C)
has few holes and few free electrons
done
clear
D)
does not have any charge carrier
done
clear
View Answer play_arrow
question_answer 7) Velocity of light is equal to:
A)
\[Y=3K(1-2\sigma )\]
done
clear
B)
\[{{I}_{1}}\]
done
clear
C)
\[{{I}_{2}}\]
done
clear
D)
\[\phi \]
done
clear
View Answer play_arrow
question_answer 8) The coefficients of mutual inductance when magnetic flux changes by \[2\times {{10}^{-2}}\,Wb\] and current changes by 0.01 A, will be:
A)
8H
done
clear
B)
4H
done
clear
C)
3H
done
clear
D)
2H
done
clear
View Answer play_arrow
question_answer 9) When a wire is stretched and its radium becomes r/2 then its resistance will be:
A)
zero
done
clear
B)
2R
done
clear
C)
8R
done
clear
D)
16R
done
clear
View Answer play_arrow
question_answer 10) If equation of a sound wave is \[y=0.0015\,\sin \,(62.8x+314t)\] then its wavelength will be:
A)
2 unit
done
clear
B)
0.3 unit
done
clear
C)
0.1 unit
done
clear
D)
0.2 unit Key Idea:
done
clear
View Answer play_arrow
question_answer 11) The latent heat of vaporization of water is 2240 J. If the work done in the process of vaporization of 1 g is 168 J, then increase in internal energy will be:
A)
1904 J
done
clear
B)
2072 J
done
clear
C)
2240 J
done
clear
D)
2408 J
done
clear
View Answer play_arrow
question_answer 12) Scent sprayer is based on:
A)
Bernoullis theorem
done
clear
B)
Archimedes principle
done
clear
C)
Charles law
done
clear
D)
Boyles law
done
clear
View Answer play_arrow
question_answer 13) If \[I={{I}_{1}}+{{I}_{2}}+2\sqrt{{{I}_{1}}{{I}_{2}}}\cos \phi \] be orbital velocity of a satellite in a circular orbital close to the earths surface and \[\cos \phi =-1\] is escape velocity from earth, then relation between the two is:
A)
\[\phi =\pi ,3\pi ,5\pi ,.................\]
done
clear
B)
\[\phi =(2\pi +1)\pi \,n=1,2,3,..............\]
done
clear
C)
\[=\frac{2\pi }{\lambda }\times \]
done
clear
D)
\[\Rightarrow \]
done
clear
View Answer play_arrow
question_answer 14) At the uppermost point of a projectile its velocity and acceleration are at an angle of:
A)
180°
done
clear
B)
90°
done
clear
C)
60°
done
clear
D)
45°
done
clear
View Answer play_arrow
question_answer 15) If the vectors \[\vec{P}=a\hat{i}+a\hat{j}+3\hat{k}\] and \[=\frac{(2n+1)\lambda }{2}\]are perpendicular to each other then the positive value of a is:
A)
zero
done
clear
B)
1
done
clear
C)
2
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 16) The speed of an electron having wavelength of \[{{10}^{-10}}m\] is:
A)
\[\frac{E}{E}=-\frac{2Q}{Q}\]
done
clear
B)
\[\Rightarrow \]
done
clear
C)
\[E=-\frac{E}{2}\]
done
clear
D)
\[3.9\Omega \]
done
clear
View Answer play_arrow
question_answer 17) Hubbles law is related with:
A)
planetary motion
done
clear
B)
speed of galaxy
done
clear
C)
black hole
done
clear
D)
comet
done
clear
View Answer play_arrow
question_answer 18) The Cauchys formula is:
A)
\[\therefore \]
done
clear
B)
\[V=E-ir\]
done
clear
C)
\[i=\frac{E}{R+r}\]
done
clear
D)
\[\therefore \]
done
clear
View Answer play_arrow
question_answer 19) How many electrons make up a charge of 20 \[V=E-\left( \frac{E}{R+r} \right)r\]C?
A)
\[1.25\,\times {{10}^{14}}\]
done
clear
B)
\[2.23\,\times {{10}^{14}}\]
done
clear
C)
\[3.25\,\times {{10}^{14}}\]
done
clear
D)
\[5.25\,\times {{10}^{14}}\]
done
clear
View Answer play_arrow
question_answer 20) The value of current gain \[\alpha \] of a transistor is 0.98. The value of \[\beta \] will be:
A)
490
done
clear
B)
4.9
done
clear
C)
59
done
clear
D)
49
done
clear
View Answer play_arrow
question_answer 21) Light of wavelength 6000\[E=2V,\,r=0.1\Omega ,R=3.9\Omega \] is reflected at nearly normal incidence from a soap films of refractive index 1.4. The least thickness of the fringe then will appear black is:
A)
infinity
done
clear
B)
200\[V=2-\left( \frac{2}{3.9+0.1} \right)\times 0.1\]
done
clear
C)
2000 \[V=1.95V\]
done
clear
D)
1000\[{{m}_{1}}{{u}_{1}}={{m}_{2}}{{v}_{2}}\]
done
clear
View Answer play_arrow
question_answer 22) A ray of light is incident on the surface of plate of glass of refractive index 1.5 at the polarizing angle. The angle of refraction of the ray will be:
A)
33.7°
done
clear
B)
43.7°
done
clear
C)
23.7°
done
clear
D)
53.7°
done
clear
View Answer play_arrow
question_answer 23) Which one of the following does not support the wave nature of light?
A)
Photoelectric effect
done
clear
B)
Interference
done
clear
C)
Polarization
done
clear
D)
Diffraction
done
clear
View Answer play_arrow
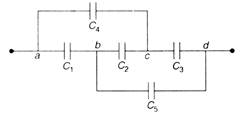
question_answer 24)
The capacitors \[{{u}_{1}}=1m/s=0.05kg,{{v}_{2}}=30m/s\]have a capacitance \[\Rightarrow \] each and \[{{C}_{2}}\] has capacitance \[{{m}_{1}}\times 1=0.05\times 30\]. The effective capacitance between a and will be:
A)
\[\Rightarrow \]
done
clear
B)
\[{{m}_{1}}=1.5kg\]
done
clear
C)
\[{{k}_{1}}\]
done
clear
D)
\[{{y}_{1}}\]
done
clear
View Answer play_arrow
question_answer 25) A siren emitting sound of frequency 800 Hz is going away from a static listener with a speed of 30 m/s. Frequency of sound to be heard by the listener is : (velocity of sound = 330 m/s)
A)
286-SHz
done
clear
B)
481.2Hz
done
clear
C)
7333Hz
done
clear
D)
644.8 Hz
done
clear
View Answer play_arrow
question_answer 26) The volume of a gas is reduced adiabatically to (1/4) of its volume at 27°C. If y = 1.4. The new temperature will be:
A)
\[{{k}_{2}}\]
done
clear
B)
\[{{y}_{2}}\]
done
clear
C)
\[y={{y}_{1}}+{{y}_{2}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 27) The velocities of sound at same temperature in two monoaromic gases densities \[{{k}_{1}}\] and \[{{F}_{1}}=-{{k}_{1}}{{y}_{1}}\] are \[{{F}_{2}}=-{{k}_{2}}{{y}_{2}}\]and \[{{k}_{2}}\] respectively, if\[{{k}_{1}}\], then, the value of \[{{k}_{2}}{{F}_{1}}+{{k}_{1}}{{F}_{2}}=-{{k}_{1}}{{k}_{2}}({{y}_{1}}+{{y}_{2}})=-{{k}_{1}}{{k}_{2}}y\] will be:
A)
4
done
clear
B)
2
done
clear
C)
\[{{F}_{1}}={{F}_{2}}=F(say)\]
done
clear
D)
\[({{k}_{1}}+{{k}_{2}})F=-{{k}_{1}}{{k}_{2}}y\]
done
clear
View Answer play_arrow
question_answer 28) The maximum range of a gun from horizontal terrain is 16 km. If \[g=10\,m/{{s}^{2}}\] what must be the muzzle velocity of the shell?
A)
400 m/s
done
clear
B)
200 m/s
done
clear
C)
100 m/s
done
clear
D)
50 m/s
done
clear
View Answer play_arrow
question_answer 29) Three different objects of masses \[F=-\frac{{{k}_{1}}{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}y\] and \[\frac{{{k}_{1}}{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}\] are allowed to fall from rest and from the same point O along three different frictionless paths. The speeds of three objects on reaching the ground will be:
A)
\[n=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}{{k}_{2}}}{({{k}_{1}}+{{k}_{2}})m}}\]
done
clear
B)
\[\frac{1}{k}=\frac{1}{{{k}_{1}}}+\frac{1}{{{k}_{2}}}\]
done
clear
C)
\[k=\frac{{{k}_{1}}{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}\]
done
clear
D)
\[n=\frac{1}{2\pi }\sqrt{\frac{k}{m}}\]
done
clear
View Answer play_arrow
question_answer 30) The radius of earth is about 6400 km and that of mars is about 3200 km. The mass of the earth is about 10 times the mass of mars. An object weighs 200 N on earths surface, then its weight on the surface of mars will be:
A)
80 N
done
clear
B)
40 N
done
clear
C)
20 N
done
clear
D)
8 N
done
clear
View Answer play_arrow
question_answer 31) A satellite is launched in co a circular orbit of radius R around the earth, while a second satellite is launched into an orbit of radius 1.01 R. The period of the second satellite is longer than the first one by approximately:
A)
3.0%
done
clear
B)
1.5%
done
clear
C)
0.7%
done
clear
D)
1.0%
done
clear
View Answer play_arrow
question_answer 32) The bulk modulus of a metal is \[{{10}^{10}}\,N/{{m}^{2}}\] and Poissons ratio 0.20. If average distance between the molecules is 3 \[=\frac{1}{2\pi }\sqrt{\frac{{{k}_{1}}{{k}_{2}}}{({{k}_{1}}+{{k}_{2}})m}}\]. then the interatomic force constant is:
A)
5.4 N/m
done
clear
B)
75 N/m
done
clear
C)
7.5 N/m
done
clear
D)
30 N/m
done
clear
View Answer play_arrow
question_answer 33) A soap bubble in vacuum has a radius 3 cm and another soap bubble in vacuum has radius 4 cm. If two bubbles coalesce under isothermal condition, then the radius of the new bubble will be:
A)
7 cm
done
clear
B)
5 cm
done
clear
C)
4.5 cm
done
clear
D)
2.3 cm
done
clear
View Answer play_arrow
question_answer 34) At what temperature the speed of sound in air will become double of its value at 27°?
A)
54° C
done
clear
B)
627 °C
done
clear
C)
927° C
done
clear
D)
327° C
done
clear
View Answer play_arrow
question_answer 35) A string in a musical instrument is 50 cm long and its fundamental frequency is 800 Hz. If the frequency of 1000 Hz is to be produced, then required length of string is:
A)
37.5cm
done
clear
B)
40cm
done
clear
C)
50cm
done
clear
D)
62.5cm
done
clear
View Answer play_arrow
question_answer 36) A conducting sphere of radius 10 cm is charged with 10 \[=\frac{Force}{Length}=\frac{[ML{{T}^{-2}}]}{[L]}=[M{{T}^{-2}}]\]C. Another uncharged sphere of radius 20 cm is allowed to touch it for some time. After that if the spheres are separated, then surface density of charges on the spheres will be in the ratio of:
A)
1 : 1
done
clear
B)
4 : 1
done
clear
C)
1 : 3
done
clear
D)
1 : 4
done
clear
View Answer play_arrow
question_answer 37) An electric bulb marked 40 W and 200 V, is used in a circuit of supply voltage 100 V. Now, its power is:
A)
10 W
done
clear
B)
20 W
done
clear
C)
40 W
done
clear
D)
100 W
done
clear
View Answer play_arrow
question_answer 38) A moving coil galvanometer is convened into an ammeter reads upto 0.03 A by connecting a shunt of resistance 4r across it and ammeter reads upto 0.06 A, when a shunt of resistance r is used. What is the maximum current which can be sent through this galvanometer if shunt is used?
A)
0.04 A
done
clear
B)
0.03 A
done
clear
C)
0.02 A
done
clear
D)
0.01 A
done
clear
View Answer play_arrow
question_answer 39) Light of wavelength \[4000\,\overset{\text{o}}{\mathop{\text{A}}}\,\] is incident on metal plate whose work function is 2 eV. What is maximum kinetic energy of emitted photoelectron?
A)
0.5 eV
done
clear
B)
1.1 eV
done
clear
C)
2.0 eV
done
clear
D)
1.5 eV
done
clear
View Answer play_arrow
question_answer 40) The flux of a-panicle at 2° is \[1\times {{10}^{6}}\]. The flux of \[[ML{{T}^{-2}}]\]-parade at angle 60° is:
A)
1.5
done
clear
B)
2.5
done
clear
C)
0.5
done
clear
D)
5.5
done
clear
View Answer play_arrow
question_answer 41) Assertion: Colored spectrum is seen when we look through a muslin cloth. Reason: It is due to the diffraction of white light on passing through fine slits.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 42) Assertion: The knowledge of Albedo helps us to estimate the atmosphere of planet. Reason: The clouds are not good reflect of light.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 43) Assertion: When a tiny circular obstacle is placed in the path of light from some distance, a bright spot is seen at the centre of shadow the obstacle. Reason: Destructive interference occur the centre of the shadow.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 44) Assertion: The quantity L/R possesses dimensions of time. Reason: To reduce the rate of increase of current through a solenoid should we increase the time constant (L/R).
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 45) Assertion: In a simple battery circuit the point of lowest potential is positive terminal of the battery. Reason: The current flows towards the point of the higher potential as it flows in such a circuit from the negative to the positive terminal.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 46) Assertion: In SHM, the motion is to and fro and periodic. Reason: Velocity of the particle, \[=[M{{L}^{-1}}{{T}^{-2}}]\] (where x is displacement)
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 47) Assertion: Stress is the internal force per unit area of a body. Reason: Rubber is more elastic than steel.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 48) Assertion: In an elastic collision of two billiard balls, the total KE is conserved during the short rime of collision of the balls (Le., when they are in contact.) Reason: Energy spent against friction does not follow the law of conservation of energy.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 49) Assertion: Thin films such as soap bubble or a thin layer of oil on water show beautiful colours when illuminated by white light. Reason: It happens due to the interference of light reflected from the upper surface of the thin film.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 50) Assertion: We cannot think of magnetic field confirmation with three poles. Reason: A bar magnet does exert a torque on itself due to its own field.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 51) Assertion: Faradays laws are consequences of conservation of energy. Reason: In a purely resistive AC circuit, the current lags behind the emf in phase.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 52) Assertion: Woollen clothes keep the body warm in winter. Reason: Air is a bad conductor of heat.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 53) Assertion: The time period of pendulum on satellite orbiting the earth is infinity. Reason: Time period of a pendulum is inversely proportional to \[[M{{L}^{2}}{{T}^{-1}}]\].
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 54) Assertion: Retardation is directly opposite to the velocity. Reason: Retardation is equal to the time rate of decrease of speed.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 55) Assertion: The dimensional formula for relative velocity is same as that of the rate of change in velocity. Reason: Relative velocity of P w.r.t. Q is the ratio of velocity of P and that of Q.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E Relative velocity of P with respect to Q = velocity of P - velocity of Q Hence, relative velocity will have dimensions of velocity. \[{{V}_{0}}\] Also rate of change of velocity is acceleration. Hence, it has dimensions of \[{{V}_{e}}\]
done
clear
View Answer play_arrow
question_answer 56) Assertion: If three capacitors of capacitances \[\alpha =\frac{\Delta {{i}_{C}}}{\Delta {{i}_{g}}}\] are connected in parallel then their equivalent capacitance \[\Delta {{i}_{C}}\] Reason: \[\Delta {{i}_{g}}\]
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 57) Assertion : First law of thermodynamics does not forbid flow of heat from lower temperature to higher temperature, Reason: Hear supplied to a system always equal to the increase in its internal energy.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 58) Assertion : Speed of wave \[\alpha =0.96,\Delta {{i}_{E}}=7.2,\] Reason: Wavelength is the distance between two nearest particles in phase.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 59) Assertion: Electric lines of force never cross each other. Reason: Electric field at a point superimpose to give one resultant electric field.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 60) Assertion: The air bubble shines in wan Reason: Air bubble in water shines due refraction of light.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 61) In Sandmeyers reaction the salt involved is:
A)
diazonium salt
done
clear
B)
cupramonium salt
done
clear
C)
ferrous salt
done
clear
D)
ammonium salt
done
clear
View Answer play_arrow
question_answer 62) Enzymes having two sites are:
A)
conjugate enzyme
done
clear
B)
apoenzyme
done
clear
C)
holoenzyme
done
clear
D)
allosteric enzyme
done
clear
View Answer play_arrow
question_answer 63) A chiral compound is:
A)
2, 3, 4- trimethyl hexane
done
clear
B)
n- hexane
done
clear
C)
methane
done
clear
D)
n-butane
done
clear
View Answer play_arrow
question_answer 64) An \[A{{B}_{2}}\] type of structure is present in:
A)
\[NaCl\]
done
clear
B)
\[{{N}_{2}}O\]
done
clear
C)
\[A{{l}_{2}}{{O}_{3}}\]
done
clear
D)
\[Ca{{F}_{2}}\]
done
clear
View Answer play_arrow
question_answer 65) The compound having highest dipole moment is:
A)
\[\underset{C{{H}_{3}}}{\overset{Cl}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{Cl}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
done
clear
B)
\[\underset{C{{H}_{3}}}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{H}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
done
clear
C)
done
clear
D)
\[\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,=\underset{H}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,\]
done
clear
View Answer play_arrow
question_answer 66) The kinetic energy of two moles of \[{{N}_{2}}\] at \[{{27}^{o}}C\] is (\[(R=8.24\,J{{K}^{-1}}mo{{l}^{-1}})\]
A)
\[5491.6\text{ }J\]
done
clear
B)
\[6491.6\text{ }J\]
done
clear
C)
\[7491.6\text{ }J\]
done
clear
D)
\[8882.4\text{ }J\]
done
clear
View Answer play_arrow
question_answer 67) The weight of one molecule of a compound \[{{C}_{60}}{{H}_{122}}\] is:
A)
\[1.3\times {{10}^{-20}}g\]
done
clear
B)
\[5.01\times {{10}^{-21}}g\]
done
clear
C)
\[3.72\times {{10}^{23}}g\]
done
clear
D)
\[1.4\times {{10}^{-21}}g\]
done
clear
View Answer play_arrow
question_answer 68) The halide having highest melting point is:
A)
\[NaF\]
done
clear
B)
\[NaCl\]
done
clear
C)
\[NaBr\]
done
clear
D)
\[NaI\]
done
clear
View Answer play_arrow
question_answer 69) At \[{{25}^{o}}C\] the pH of a solution containing 0.10 M sodium acetate and 0.03 M acetic acid is: [\[_{p}{{K}_{a}}\] value of \[C{{H}_{3}}COOH=4.57\]]
A)
\[3.24\]
done
clear
B)
\[4.59\]
done
clear
C)
\[5.09\]
done
clear
D)
\[6.67\]
done
clear
View Answer play_arrow
question_answer 70) The enthalpy of formation of ammonia is \[=46.0\,kJ\,mo{{l}^{-1}}\]. The enthalpy change for following reaction is : \[2N{{H}_{3}}\xrightarrow{{}}{{N}_{2}}+3{{H}_{2}}\]
A)
42.0kJ
done
clear
B)
64.0kJ
done
clear
C)
86.0kJ
done
clear
D)
92.0kJ
done
clear
View Answer play_arrow
question_answer 71) The molecule having highest bond energy is:
A)
\[N-N\]
done
clear
B)
\[F-F\]
done
clear
C)
\[C-C\]
done
clear
D)
\[O-O\]
done
clear
View Answer play_arrow
question_answer 72) Liquid ammonia and liquor ammonia are:
A)
same
done
clear
B)
different
done
clear
C)
allotropes
done
clear
D)
isotopes
done
clear
View Answer play_arrow
question_answer 73) Thermite is a mixture of iron oxide and:
A)
aluminium powder
done
clear
B)
zinc powder
done
clear
C)
potassium metal
done
clear
D)
sodium metal
done
clear
View Answer play_arrow
question_answer 74) The size of\[C-C\] bond in benzene is:
A)
\[1.54\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[1.39\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[1.22\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[1.21\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 75) Hydrogen is produced by the reaction:
A)
\[N{{a}_{2}}{{O}_{2}}+2HCl\]
done
clear
B)
\[Mg+{{H}_{2}}O\]
done
clear
C)
\[Ba{{O}_{2}}+HCl\]
done
clear
D)
\[{{H}_{2}}{{S}_{4}}{{O}_{8}}+{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 76) At \[{{80}^{o}}C({{H}_{3}}{{O}^{+}})\] of distilled water is \[{{10}^{-6}}\] mol/L. At the same temperature the value of \[{{K}_{w}}\] is:
A)
\[1\times {{10}^{-3}}\]
done
clear
B)
\[1\times {{10}^{-6}}\]
done
clear
C)
\[1\times {{10}^{-9}}\]
done
clear
D)
\[1\times {{10}^{-12}}\]
done
clear
View Answer play_arrow
question_answer 77) Which of the following is an iron ore?
A)
Cassiterite
done
clear
B)
Magnetite
done
clear
C)
Galena
done
clear
D)
Copper pyrite
done
clear
View Answer play_arrow
question_answer 78) Propyne and propene can be distinguished by:
A)
dil. \[KMn{{O}_{4}}\]
done
clear
B)
conc. \[{{H}_{2}}S{{O}_{4}}\]
done
clear
C)
\[AgN{{O}_{3}}\] in ammonia
done
clear
D)
\[B{{r}_{2}}\] in \[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 79) The number of a and n bonds present in pent-1- ene-4-yne is:
A)
3, 10
done
clear
B)
9, 4
done
clear
C)
4, 9
done
clear
D)
10, 3
done
clear
View Answer play_arrow
question_answer 80) Acetylene and dil. \[{{H}_{2}}S{{O}_{4}}\] reacts to produce:
A)
\[C{{H}_{3}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 81) \[{{C}_{2}}{{H}_{5}}OH+SOC{{l}_{2}}\xrightarrow{pyridine}\] \[{{C}_{2}}{{H}_{5}}Cl+S{{O}_{2}}+HCl\] The above reaction is known as:
A)
Williamsons synthesis
done
clear
B)
Hoffmanns reaction
done
clear
C)
Mendius reaction
done
clear
D)
Darzens process
done
clear
View Answer play_arrow
question_answer 82) Methyl orange comes under:
A)
mordant dye
done
clear
B)
nitro dye
done
clear
C)
acid dye
done
clear
D)
basic dye
done
clear
View Answer play_arrow
question_answer 83) Cyanohydrin can easily be prepared from:
A)
\[{{C}_{2}}{{H}_{5}}-{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}COOH\]
done
clear
C)
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}CO{{C}_{2}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 84) Quantum numbers of an atom can be defined on the basis of:
A)
Aufbaus principle
done
clear
B)
Heisenbergs uncertainty principle
done
clear
C)
Hunds rule
done
clear
D)
Paulis exclusion principle
done
clear
View Answer play_arrow
question_answer 85) The half-life of a substance in a first order reaction is 15 min. The rate constant is:
A)
\[2.46\times {{10}^{2}}\text{ }mi{{n}^{-1}}\]
done
clear
B)
\[4.62\times {{10}^{-2}}{{\min }^{-1}}\]
done
clear
C)
\[6.74\times {{10}^{-2}}{{\min }^{-1}}\]
done
clear
D)
\[7.18\times {{10}^{2}}{{\min }^{-1}}\]
done
clear
View Answer play_arrow
question_answer 86) The tribasic acid is:
A)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
C)
\[{{H}_{3}}P{{O}_{2}}\]
done
clear
D)
\[{{H}_{4}}{{P}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 87) Oxidation number of Fe in \[F{{e}_{3}}{{O}_{4}}\] is:
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{2}{6}\]
done
clear
C)
\[\frac{8}{3}\]
done
clear
D)
\[\frac{3}{2}\]
done
clear
View Answer play_arrow
question_answer 88) Which of the electronic configuration shows noble gas?
A)
\[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\]
done
clear
B)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{3}}\]
done
clear
C)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{1}}\]
done
clear
D)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{4}}\]
done
clear
View Answer play_arrow
question_answer 89) Acetone can easily be converted into propane by the action of:
A)
\[HI\]
done
clear
B)
\[{{H}_{3}}P{{O}_{3}}\]
done
clear
C)
\[HN{{O}_{3}}\]
done
clear
D)
\[HI{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 90) 1000 g calcium carbonate solution contains 10g carbonate. The concentration of solution is:
A)
\[10ppm\]
done
clear
B)
\[100ppm\]
done
clear
C)
\[1000ppm\]
done
clear
D)
\[10,000ppm\]
done
clear
View Answer play_arrow
question_answer 91) The monomer of teflon is:
A)
tetra chloroethylene
done
clear
B)
tetra bromoethylene
done
clear
C)
tetra iodoethylene
done
clear
D)
tetra fluoroethylene
done
clear
View Answer play_arrow
question_answer 92) The most stable ion is:
A)
\[C{{H}_{3}}\overset{+}{\mathop{C{{H}_{2}}}}\,\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}\overset{+}{\mathop{CH}}\,\]
done
clear
C)
\[{{(C{{H}_{3}})}_{3}}{{C}^{+}}\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}\overset{+}{\mathop{C{{H}_{2}}}}\,\]
done
clear
View Answer play_arrow
question_answer 93) The IUPAC name of following compound is: \[C{{H}_{3}}-C{{(C{{H}_{3}})}_{2}}-CH=C{{(C{{H}_{3}})}_{2}}\]
A)
1, 1, 3, 3-tetramethyl-but-1- ene
done
clear
B)
1, 3, 3-trimethyl-pent-2-ene
done
clear
C)
2, 2, 4-trimethyl but-4-ene
done
clear
D)
2, 4, 4-trimethyl pent-2-ene
done
clear
View Answer play_arrow
question_answer 94) \[{{H}_{2}}+C{{l}_{2}}\xrightarrow{Sunlight}2HCl\] In the above reaction the order of reaction is:
A)
3
done
clear
B)
2
done
clear
C)
0
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 95) The mass of 70% \[{{H}_{2}}S{{O}_{4}}\] required for neutralization of one mole of \[NaOH\] is:
A)
70 g
done
clear
B)
35 g
done
clear
C)
30 g
done
clear
D)
95 g
done
clear
View Answer play_arrow
question_answer 96) Schottky defect defines imperfection in the lattice structure of a:
A)
gas
done
clear
B)
plasma
done
clear
C)
liquid
done
clear
D)
solid
done
clear
View Answer play_arrow
question_answer 97) A compound possess 8% sulphur by mass. The least molecular mass is:
A)
200
done
clear
B)
400
done
clear
C)
155
done
clear
D)
355
done
clear
View Answer play_arrow
question_answer 98) The solubility of \[AgCl\] in \[0.2\text{ }M\,NaCl\] is: \[[{{K}_{sp}}AgCl=1.8\times {{10}^{-10}}]\]
A)
\[1.8\times {{10}^{-11}}M\]
done
clear
B)
\[9\times {{10}^{-10}}M\]
done
clear
C)
\[6.5\times {{10}^{-12}}M\]
done
clear
D)
\[5.6\times {{10}^{-11}}M\]
done
clear
View Answer play_arrow
question_answer 99) Lucas test is used for:
A)
aldehydes
done
clear
B)
alkyl halides
done
clear
C)
alcohols
done
clear
D)
acids
done
clear
View Answer play_arrow
question_answer 100) Phenol \[\xrightarrow[{{H}^{+}}]{CHC{{l}_{3}}/NaOH}\] salicylaldehyde The; above reaction is known as:
A)
Reimer-Tiemann reaction
done
clear
B)
Bucherer reaction
done
clear
C)
Gattermann synthesis
done
clear
D)
Perkin reaction
done
clear
View Answer play_arrow
question_answer 101) Assertion: Stannous chloride is a powerful oxidising agent which oxidises mercuric chloride to mercury. Reason: Stannous chloride gives grey precipitate with mercuric chloride, but stannic chloride does not do so.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 102) Assertion: Physical absorption of molecules takes place on surface only. Reason: In this process, the bonds of the absorbed molecules are broken.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 103) Assertion: The fluorine has lower reactivity. Reason: \[F-F\] bond has low bond dissociation energy.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 104) Assertion: Absolute values of internal energy of substance cannot be determined; Reason: It is impossible to determine exact valuer or constituent energies of the substances.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 105) Assertion: Mass and volume are extensive properties. Reason: Mass/volume is also an extensive parameter.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 106) Assertion: Work done by an ideal gas during reversible isothermal expansion is zero. Reason: In vacuum external pressure against which expansion occur is zero.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 107) Assertion: DNA molecules and RNA molecules are found in the nucleus of a cell. Reason: On heating the enzyme do not lose their specific activity.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 108) Assertion: Halogens absorb visible light. Reason: All halogens are coloured.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 109) Assertion: The first ionisation energy of Be is greater than boron. Reason: 2p orbitals have lower energy than 25 orbitals.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 110) Assertion: K and Cs are used in photo-electric cells. Reason: K and Cs emit electrons on exposure to light.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 111) Assertion: \[\sigma \] is strong while n is a weak bond. Reason: Atoms rotate freely about n bond.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 112) Assertion: A beam of electrons deflects more than a beam of a-particles in an electric field. Reason: Electrons possess negative charge while \[\alpha \] -particles possess positive charge,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 113) Assertion: -Alcohol and phenol can be distinguished by sodium hydroxide. Reason: Phenol is acidic while alcohol is neutral.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 114) Assertion: Acetophenone and benzop- henone can be distinguished byiodoform test. Reason: Acetophenone and benzophenone both are carbonyl compounds.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 115) Assertion: Potassium ferrocyanide and potassium ferricyanite both are diamagnetic. Reason: Both have unpaired electrons.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 116) Assertion: Benzene is reactive while inorganic benzene is unreactive compound. Reason: Inorganic benzene is borazine, \[{{B}_{3}}{{N}_{3}}{{H}_{6}}\].
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 117) Assertion: Isotonic solution do not show the phenomenon of osmosis. Reason: Isotonic solutions have equal osmotic pressure.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 118) Assertion: \[CC{{l}_{4}}\] and \[{{H}_{2}}O\] are immiscible. Reason: \[CC{{l}_{4}}\] is a polar solvent.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 119) Assertion: All amino acids exist as Zwitter ions. Reason: Amino acids have both \[-N{{H}_{2}}\] and \[-COOH\] group.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 120) Assertion: o and p-nitrophenols can be separated by steam distillation. Reason: o-nitrophenol have intra-molecular hydrogen bonding while p-nitrophenol exists as associated molecules.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 121) Frame shift mutation occurs when:
A)
base is added
done
clear
B)
base is deleted
done
clear
C)
base is added or deleted
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 122) A chromosome with centromere at one end is called:
A)
telocentric
done
clear
B)
metacentric
done
clear
C)
excentric
done
clear
D)
apocentric
done
clear
View Answer play_arrow
question_answer 123) In glycolysis, glucose molecule is converted in to:
A)
PEP
done
clear
B)
RuBP
done
clear
C)
acetyl Co-A
done
clear
D)
pyruvic acid
done
clear
View Answer play_arrow
question_answer 124) Algae are useful because they:
A)
purify the atmosphere
done
clear
B)
are large in number
done
clear
C)
are used in fermentation
done
clear
D)
are used to study respiration
done
clear
View Answer play_arrow
question_answer 125) Introduction of foreign gene for improving genotype is called:
A)
tissue culture
done
clear
B)
vernalization
done
clear
C)
genetic engineering
done
clear
D)
eugenics
done
clear
View Answer play_arrow
question_answer 126) Pure line breed refers to:
A)
homozygosity
done
clear
B)
heterozygosity
done
clear
C)
linkage
done
clear
D)
both (b) and (c)
done
clear
View Answer play_arrow
question_answer 127) If a homozygous red flowered plant is crossed with a homozygous white flowered plant, the offsprings would be:
A)
all red flowered
done
clear
B)
half red flowered
done
clear
C)
half white flowered
done
clear
D)
all white flowered
done
clear
View Answer play_arrow
question_answer 128) Bud dormancy can be induced by:
A)
IAA
done
clear
B)
GA
done
clear
C)
ABA
done
clear
D)
Ethylene
done
clear
View Answer play_arrow
question_answer 129) Xenia refers to effect of pollen on:
A)
endosperm
done
clear
B)
stems
done
clear
C)
taste of fruits
done
clear
D)
vascular tissue
done
clear
View Answer play_arrow
question_answer 130) The plant body of Funaria is:
A)
sporophyte
done
clear
B)
gametophyte
done
clear
C)
predominantly sporophyte with independent gametophyte
done
clear
D)
predominantly gametophyte with dependent sporophyte
done
clear
View Answer play_arrow
question_answer 131) Elaters help in dispersal of spores of:
A)
Riccia
done
clear
B)
Marchantia
done
clear
C)
Dryopteris
done
clear
D)
Funaria
done
clear
View Answer play_arrow
question_answer 132) The nature of megasporophyll of Cycas is similar to:
A)
stamen
done
clear
B)
carpel
done
clear
C)
sepal
done
clear
D)
petal
done
clear
View Answer play_arrow
question_answer 133) Which of following type of anther is found in Malvaceae?
A)
Monothecous
done
clear
B)
Dithecous
done
clear
C)
Polythecous
done
clear
D)
Without thecous
done
clear
View Answer play_arrow
question_answer 134) Which of the following helps in respiration of Lichens?
A)
Isidia
done
clear
B)
Soredia
done
clear
C)
Cyphella
done
clear
D)
Cephalodia
done
clear
View Answer play_arrow
question_answer 135) Green mufler is useful against:
A)
air pollution
done
clear
B)
noise pollution
done
clear
C)
soil pollution
done
clear
D)
radioactive pollution
done
clear
View Answer play_arrow
question_answer 136) Passage cells are found in:
A)
endodermis
done
clear
B)
pericycle
done
clear
C)
cortex
done
clear
D)
epiblemma
done
clear
View Answer play_arrow
question_answer 137) Which of the following organelle is related with photorespiration?
A)
Peroxisome
done
clear
B)
Chloroplast
done
clear
C)
Mitochondria
done
clear
D)
Lysosome
done
clear
View Answer play_arrow
question_answer 138) Proteins are:
A)
polysaccharides
done
clear
B)
polyamides
done
clear
C)
polynucleotides
done
clear
D)
polyglycol
done
clear
View Answer play_arrow
question_answer 139) Wobble hypothesis was given by:
A)
F.H.C. Crick
done
clear
B)
Nirenberg
done
clear
C)
Holley
done
clear
D)
Khorana
done
clear
View Answer play_arrow
question_answer 140) Fascicular cambium is the cambium of vascular bundle of:
A)
monocot stem
done
clear
B)
dicot stem
done
clear
C)
monocot leaf
done
clear
D)
dicot leaf
done
clear
View Answer play_arrow
question_answer 141) Mesophyll is usually differentiated in:
A)
monocot leaf
done
clear
B)
isobilateral leaf
done
clear
C)
dorsiventral leaf
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 142) Which of the following gives fehlings test?
A)
Pectin
done
clear
B)
Sucrose
done
clear
C)
Cellulose
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 143) The nicotinamide is synthesized in our body from:
A)
tryptophan
done
clear
B)
trvosine
done
clear
C)
valine
done
clear
D)
alanine
done
clear
View Answer play_arrow
question_answer 144) Curdling of milk in small intestine takes place due to:
A)
rennin
done
clear
B)
trypsin
done
clear
C)
chymotrypsin
done
clear
D)
ptylase
done
clear
View Answer play_arrow
question_answer 145) Lysis of foreign cell is mediated through
A)
IgM
done
clear
B)
IgA
done
clear
C)
IgE
done
clear
D)
IgM and lgG
done
clear
View Answer play_arrow
question_answer 146) Which of the following has minimum pH?
A)
Bile
done
clear
B)
Saliva
done
clear
C)
Gastric juice
done
clear
D)
Pancreatic juice
done
clear
View Answer play_arrow
question_answer 147) The places of first, second and third moulting of Ascaris larva are:
A)
soil, alveoli, lung
done
clear
B)
liver, soil, stomad
done
clear
C)
soil, lung, liver
done
clear
D)
soil, intestine,
done
clear
View Answer play_arrow
question_answer 148) Which of following teeth are lophodont?
A)
Incisor and canine
done
clear
B)
Premolar and molar
done
clear
C)
Canine and premolar
done
clear
D)
Premolar and incisor
done
clear
View Answer play_arrow
question_answer 149) Cyclosporine is used:
A)
for allergy
done
clear
B)
as immunodepressent
done
clear
C)
prophylactic for virus
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 150) What is left, when bath sponges dries up?
A)
Spicules
done
clear
B)
Hold fast
done
clear
C)
Spongin fibres
done
clear
D)
Tentacles
done
clear
View Answer play_arrow
question_answer 151) Hydra receives impulses and stimu through:
A)
nerve cells
done
clear
B)
sensory cells
done
clear
C)
neuron cell
done
clear
D)
nematocysts
done
clear
View Answer play_arrow
question_answer 152) Adults of Wauchereria bancrofti attacks:
A)
kidney
done
clear
B)
heart
done
clear
C)
nervous system
done
clear
D)
lymph vessel
done
clear
View Answer play_arrow
question_answer 153) Ploidy of ovum of angiosperms is:
A)
haploid
done
clear
B)
diploid
done
clear
C)
triploid
done
clear
D)
polyploid
done
clear
View Answer play_arrow
question_answer 154) Anaerobic respiration, after glycolysis is also called as:
A)
fermentation
done
clear
B)
fragmentation
done
clear
C)
restoration
done
clear
D)
multiplication
done
clear
View Answer play_arrow
question_answer 155) Which of the following are uricotelic animals?
A)
Rohu, frog
done
clear
B)
Camel, frog
done
clear
C)
Lizard, crow
done
clear
D)
Eagles, earthworm
done
clear
View Answer play_arrow
question_answer 156) Sharpeys perforating fibers are related with:
A)
heart contraction
done
clear
B)
muscle relaxation
done
clear
C)
fixing of teeth
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 157) Whartons duct is the duct of:
A)
parotid gland
done
clear
B)
submandibular salivary gland
done
clear
C)
submaxillary gland
done
clear
D)
pancreatic gland
done
clear
View Answer play_arrow
question_answer 158) In Entamoeba histolytica, the presence of chromatid bodies is characteristic of:
A)
precystic stage
done
clear
B)
trophozoite stage
done
clear
C)
mature binucleate stage
done
clear
D)
both (a) and (b)
done
clear
View Answer play_arrow
question_answer 159) The cranial capacity was largest among them:
A)
Peking man
done
clear
B)
African man
done
clear
C)
Java ape man
done
clear
D)
Neanderthal man
done
clear
View Answer play_arrow
question_answer 160) MO. The phagocytosis was first of all seen by:
A)
Huxley
done
clear
B)
Haeckel
done
clear
C)
Matchnikoff
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 161) Assertion: Collenchyma is thick walled dead tissue. Reason: Collenchymatous cells show thickenings of pectin.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 162) Assertion: Bacterial photosynthesis occurs by utilizing wavelength longer than 700 nm. Reason: Here reaction center is P-890.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 163) Assertion: The two cotyledons in seed are embryonic leaves. Reason: The embryo contain radicle and plumule.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 164) Assertion: Systematics is the branch of biology that deals with classification of living organisms. Reason: The aim of classification is to group the organisms.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 165) Assertion: The megaspore mother cell divide mitotically to produce four spores. Reason: Megaspore mother cells are diploid and megaspore is haploid.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 166) Assertion: Six molecules of \[C{{O}_{2}}\] and twelve molecules of \[NADP{{H}^{+}}+{{H}^{+}}\] and 18 ATP are used to form one hexose molecules. Reason: Light reaction results in formation of ATP and \[NADP{{H}_{2}}\].
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 167) Assertion: Plasmids are single-stranded extra chromosomal DNA. Reason: Plasmids are usually present in eukaryotic cells.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 168) Assertion: m-RNA attaches to ribosome through its 3 end. Reason: The m-RNA has F-capsular nucleotide and bases of lagging sequence,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 169) Assertion: Organisms are made up of cells. Reason: Cell are structural unit of living organisms. A cell keeps its chemical composition steady within its boundary.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 170) Assertion: Clones are produced by sexual reproduction and same sexual process. Reason: These are prepared by group of cells descended from many cells or by inbreeding of a heterozygous line.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 171) Assertion: Living organisms are regarded as closed systems. Reason: Energy of living organisms cannot be lost or gained from external environment,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 172) Assertion: Death is one of the important regulatory process on earth. Reason: It avoids over-crowding caused by continuous reproduction.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 173) Assertion: The imbalance in concentration of \[N{{a}^{+}},\,{{K}^{+}}\] and proteins generates resting potential. Reason: To maintain the unequal distribution of \[N{{a}^{+}}\] and \[{{K}^{+}}\], the neurons use electrical energy.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 174) Assertion: The regulation of RBC production is accomplished by FSH. Reason: Erythropoietin hormone circulates to red bone marrow where it increases stem cell mitosis and speed up development of RBCs.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 175) Assertion: WBCs accumulate at site of wounds by diapedesis. Reason: It is squeezing of leucocytes from endothelium.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 176) Assertion: Specialization of cells is useful organism. Reason: it increases the operational efficiency of an organism.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 177) Assertion: The number of cells in multicellular organism is inversely proportional to size of body. Reason: All cells of biological world alive.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 178) Assertion: Histamine is related with allergic and inflammatory reactions. Reason: Histamine is a vasodilator,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 179) Assertion: Lateral line system is found fishes and aquatic larval amphibians. Reason: Lateral line system has receptor sensory cells derived from ectoderm,
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 180) Assertion: Glycolysis occurs in cytoplasm. Reason: Enzymes of glycolysis are found in cytoplasm. It is common in aerobic/anaerobic respiration.
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
D
done
clear
E)
E
done
clear
View Answer play_arrow
question_answer 181) Gayatri Mantra is related with:
A)
AtharVeda
done
clear
B)
Rig Veda
done
clear
C)
YajurVeda
done
clear
D)
Sam Veda
done
clear
View Answer play_arrow
question_answer 182) Who was chosen Time Magazines Person for the year 2001?
A)
Collin Powel
done
clear
B)
Mike Monore
done
clear
C)
George Bush
done
clear
D)
Rudolf Guilani
done
clear
View Answer play_arrow
question_answer 183) How many languages are recognize by the Constitution of India in the 8th schedule?
A)
12
done
clear
B)
14
done
clear
C)
16
done
clear
D)
18
done
clear
View Answer play_arrow
question_answer 184) Who is known as the Iron Man of India?
A)
Jawaharlal Nehru
done
clear
B)
Bal Gangadhar Tilak
done
clear
C)
Sardar Vallabhbhai Patel
done
clear
D)
Mahatma Gandhi
done
clear
View Answer play_arrow
question_answer 185) Which of the following Hindi Indian movies was nominated in the category of foreign language film for Oscar Award 2002?
A)
Mansoon Wedding
done
clear
B)
Dil Chahata Hai
done
clear
C)
Gadar Ek Prem Katha
done
clear
D)
Lagaan
done
clear
View Answer play_arrow
question_answer 186) The founder of Khalsa was:
A)
Guru Gobind Singh
done
clear
B)
Guru Nanak Dev
done
clear
C)
Guru Ram Das
done
clear
D)
Guru TeghBahadur
done
clear
View Answer play_arrow
question_answer 187) The design of the National Flag was adopted by the constituent assembly of India on:
A)
26th January, 1949
done
clear
B)
26th January, 1950
done
clear
C)
22nd July, 1947
done
clear
D)
15th August, 1947
done
clear
View Answer play_arrow
question_answer 188) Who was the last Viceroy of India?
A)
Lord David
done
clear
B)
Lord Wavell
done
clear
C)
Lord Mountbatten
done
clear
D)
Wellington
done
clear
View Answer play_arrow
question_answer 189) When was the first football world cup held?
A)
1930
done
clear
B)
1950
done
clear
C)
1954
done
clear
D)
1968
done
clear
View Answer play_arrow
question_answer 190) Human Organ Development Centre for Transplantation is going to be established in India at:
A)
Vellore
done
clear
B)
Mumbai
done
clear
C)
Hyderabad
done
clear
D)
Chennai
done
clear
View Answer play_arrow
question_answer 191) When was the golden jubilee of Indian Parliament celebrated?
A)
1st January, 1997
done
clear
B)
26th January, 2002
done
clear
C)
13th May, 2002
done
clear
D)
15th August, 1997
done
clear
View Answer play_arrow
question_answer 192) An Indian river, that does not form any delta is:
A)
Cauvery
done
clear
B)
Narmada
done
clear
C)
Yamuna
done
clear
D)
Singh
done
clear
View Answer play_arrow
question_answer 193) How many islands are there in Lakshadweep?
A)
47
done
clear
B)
36
done
clear
C)
27
done
clear
D)
17
done
clear
View Answer play_arrow
question_answer 194) July 11th is celebrated as:
A)
Doctors Day
done
clear
B)
Van Mahotsava Day
done
clear
C)
AIDS Day
done
clear
D)
World Population Day
done
clear
View Answer play_arrow
question_answer 195) Mens Single US Open, 2001 Championship won by:
A)
Leyton Herwitt
done
clear
B)
Pete Sampras
done
clear
C)
Safin
done
clear
D)
Stefan Edberg
done
clear
View Answer play_arrow
question_answer 196) Which one of the classical dance forms originated in Andhra Pradesh?
A)
Odissi
done
clear
B)
Kathakali
done
clear
C)
Kuchipudi
done
clear
D)
Bharat Natyam
done
clear
View Answer play_arrow
question_answer 197) The spinning of the earth on its imaginary axis is known as :
A)
rotation
done
clear
B)
circulation
done
clear
C)
orbiting
done
clear
D)
revolution
done
clear
View Answer play_arrow
question_answer 198) Who is CEAT International Cricketer of the year 2000-2001?
A)
Sachin Tendulkar
done
clear
B)
Muttiah Muralitharan
done
clear
C)
Shane Wame
done
clear
D)
Brayan Lara
done
clear
View Answer play_arrow
question_answer 199) Which organ of the body purifies the blood?
A)
Heart
done
clear
B)
Lungs
done
clear
C)
Kidneys
done
clear
D)
Pancreas
done
clear
View Answer play_arrow
question_answer 200) Who is called Nightingale of India?
A)
Indira Gandhi
done
clear
B)
Lata Mangeshker
done
clear
C)
Asha Bhonsle
done
clear
D)
Sarojini Naidu
done
clear
View Answer play_arrow