A) 2
B) 4
C) 6
D) 8
Correct Answer: C
Solution :
Key Idea: A diatomic molecule can rotate about any of three co-ordinate axes. The molecule of a triatomic gas has a tendency of rotating about any of three co-ordinate axes. So it has 6 degrees of freedom, 3 translational and 3 rotational. At high enough temperature a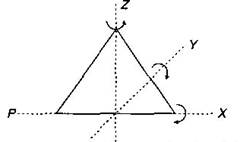 triatomic molecule has 2 vibrational degrees of freedom. But as temperature requirement is not given, so we answer simply by assuming triatomic gas molecule at room temperature. Thus, f = 6 (3 translational + 3 rotational) at room temperature. Alternative: For non linear triatomic gas, N = 3 and restrictions k are also 3.
triatomic molecule has 2 vibrational degrees of freedom. But as temperature requirement is not given, so we answer simply by assuming triatomic gas molecule at room temperature. Thus, f = 6 (3 translational + 3 rotational) at room temperature. Alternative: For non linear triatomic gas, N = 3 and restrictions k are also 3.  \[\therefore \] \[f=3N-k\] \[=3\times 3-3=6\] For linear triatomic gas \[k=2\] \[\therefore \] \[f=3\times 3-2=7\]
\[\therefore \] \[f=3N-k\] \[=3\times 3-3=6\] For linear triatomic gas \[k=2\] \[\therefore \] \[f=3\times 3-2=7\]
You need to login to perform this action.
You will be redirected in
3 sec
