A) II < III > I
B) I > II > III
C) III > II > I
D) II > III > I
Correct Answer: B
Solution :
In aniline \[-N{{H}_{2}}\]group is attached with benzene ring. \[-N{{H}_{2}}\]group shows +M effect. So it activates the benzene ring. Hence, rate of electrophilic substitution is increased due to increase in the electron density at o/p position. In case of nitrobenzene, \[-N{{O}_{2}}\]deactivates the benzene ring, so in nitrobenzene rate of electrophilic substitution is lower than benzene. Hence order of \[{{S}_{E}}\] reaction: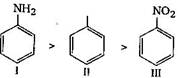
You need to login to perform this action.
You will be redirected in
3 sec
