A)
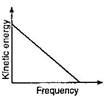
B)
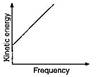
C)
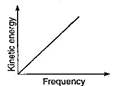
D)
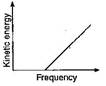
Correct Answer: D
Solution :
Key Idea: Compare the Einstein's photo-electric equation with the equation of straight line. Einstein's photoelectric equation is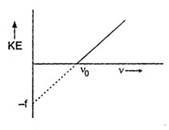 where\[\phi =\] work function of metal Comparing above Eq. (i) with equation of a straight line \[y=mx+c\] we get \[m=h,\,\,\,c=-\phi \] Therefore, if we draw a graph between kinetic energy and frequency, then a straight line cutting the frequency axis at \[{{v}_{0}}\]and giving an intercept of \[(-\phi )\]on the kinetic energy axis, is obtained.
where\[\phi =\] work function of metal Comparing above Eq. (i) with equation of a straight line \[y=mx+c\] we get \[m=h,\,\,\,c=-\phi \] Therefore, if we draw a graph between kinetic energy and frequency, then a straight line cutting the frequency axis at \[{{v}_{0}}\]and giving an intercept of \[(-\phi )\]on the kinetic energy axis, is obtained.
You need to login to perform this action.
You will be redirected in
3 sec
