A) \[{{\mu }_{d}}\ne 0\,\,and\,\,{{\mu }_{f}}\ne 0\]
B) \[{{\mu }_{p}}=0\,and\,{{\mu }_{f}}\ne 0\]
C) \[{{\mu }_{d}}=0\,\,and\,\,{{\mu }_{p}}\ne 0\]
D) \[{{\mu }_{d}}\ne 0\,and\,{{\mu }_{p}}=0\]
Correct Answer: C
Solution :
In diamagnetic substances in each pair of electrons, the spin of both the electrons are in opposite directions. Hence, the electrons of each pair completely cancel the magnetic moment of each other. Thus, the net magnetic moment of each atom of such substances is zero i.e., \[{{\mu }_{d}}=0\].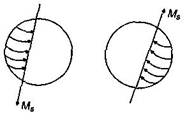 The property of paramagnetism is found in those substances whose atoms, or molecules have an excess of electrons spinning in same direction. Hence, atoms of paramagnetic substances have permanent magnetic moment i.e., \[{{\mu }_{p}}\ne 0.\] The property of ferromagnetism is found in substances which acquire very strong magnetism when placed in an external magnetic field. Like the paramagnetic substances each atom of ferromagnetic substances also has a permanent magnetic moment i.e. \[i.e.,\,{{\mu }_{f}}\ne 0\].
The property of paramagnetism is found in those substances whose atoms, or molecules have an excess of electrons spinning in same direction. Hence, atoms of paramagnetic substances have permanent magnetic moment i.e., \[{{\mu }_{p}}\ne 0.\] The property of ferromagnetism is found in substances which acquire very strong magnetism when placed in an external magnetic field. Like the paramagnetic substances each atom of ferromagnetic substances also has a permanent magnetic moment i.e. \[i.e.,\,{{\mu }_{f}}\ne 0\]. 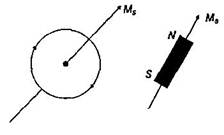
You need to login to perform this action.
You will be redirected in
3 sec
