A) \[{{[Zn\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
B) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
D) \[{{[Ni\,{{(N{{H}_{3}})}_{6}}]}^{2+}}\]
Correct Answer: C
Solution :
In [Co(NH3)6]3+, oxidation state of Co = + 3 and its co-ordination number is six. So \[_{27}Co=1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3{{p}^{6}},3{{d}^{7}},4{{s}^{2}}\] \[C{{o}^{3+}}=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}\]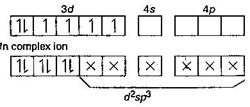 In \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] shows inner orbital complex as well as diamagnetic in behaviour (due to absence of upaired electron). \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{3+}}\] \[\to \] \[s{{p}^{3}}{{d}^{2}}\] hybridisation (outer) and diamagnetic. \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{2+}}\to \,{{d}^{2}}s{{p}^{3}}\] hybridisation (inner) and paramagnetic.
In \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] shows inner orbital complex as well as diamagnetic in behaviour (due to absence of upaired electron). \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{3+}}\] \[\to \] \[s{{p}^{3}}{{d}^{2}}\] hybridisation (outer) and diamagnetic. \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{2+}}\to \,{{d}^{2}}s{{p}^{3}}\] hybridisation (inner) and paramagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
