A) less than \[\Delta H\]
B) equal to \[\Delta H\]
C) more than \[\Delta H\]
D) equal to zero
Correct Answer: C
Solution :
Key Idea In endothermic reactions, energy of reactants is less than that of the products. Potential energy diagram for endothermic reactions is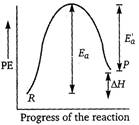 where,\[{{E}_{a}}=\] activation energy of forward reaction \[E{{'}_{a}}=\] activation energy of backward reaction \[\Delta H=\] enthalpy of the reaction From the above diagram, \[{{E}_{a}}=E_{a}^{'}+\Delta H\] Thus, \[{{E}_{a}}>\Delta H\]
where,\[{{E}_{a}}=\] activation energy of forward reaction \[E{{'}_{a}}=\] activation energy of backward reaction \[\Delta H=\] enthalpy of the reaction From the above diagram, \[{{E}_{a}}=E_{a}^{'}+\Delta H\] Thus, \[{{E}_{a}}>\Delta H\]
You need to login to perform this action.
You will be redirected in
3 sec
