A) \[-1.8{{\Delta }_{o}}\]
B) \[-1.6{{\Delta }_{o}}+P\]
C) \[-1.2{{\Delta }_{o}}\]
D) \[-0.6{{\Delta }_{o}}\]
Correct Answer: D
Solution :
Key Idea In case of high spin complex, \[{{\Delta }_{o}}\] is small. Thus, the energy required to pair up the fourth electron with the electrons of lower energy d-orbitals would be higher than that required to place the electrons in the higher d-orbital. Thus, pairing does not occur. For high spin \[{{d}^{4}}\] octahedral complex,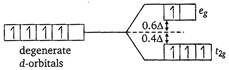 \[\therefore \]Crystal field stabilization energy \[=(-3\times 0.4+1\times 0.6){{\Delta }_{o}}\] \[=(-1.2+0.6)\,{{\Delta }_{o}}\] \[=-0.6\,{{\Delta }_{o}}\]
\[\therefore \]Crystal field stabilization energy \[=(-3\times 0.4+1\times 0.6){{\Delta }_{o}}\] \[=(-1.2+0.6)\,{{\Delta }_{o}}\] \[=-0.6\,{{\Delta }_{o}}\]
You need to login to perform this action.
You will be redirected in
3 sec
