A) \[C{{l}_{2}}O<Cl{{O}_{2}}<ClO_{2}^{-}\]
B) \[C{{l}_{2}}O<C{{l}_{2}}O<ClO_{2}^{-}\]
C) \[C{{l}_{2}}O<ClO_{2}^{-}<ClO_{2}^{{}}\]
D) \[ClO_{2}^{-}<C{{l}_{2}}O<ClO_{2}^{{}}\]
Correct Answer: D
Solution :
Key Idea As the number of tone pairs of electrons increases, bond angle decreases due to repulsion between Ip - Ip. Moreover, as the electro negativity of central atom decreases, bond angle decreases. Hence, the order of bond angle is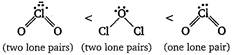 (Cl is less electronegative as compared to O.)
(Cl is less electronegative as compared to O.)
You need to login to perform this action.
You will be redirected in
3 sec
