A)
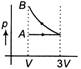
B)
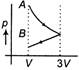
C)
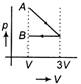
D)
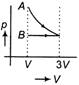
Correct Answer: D
Solution :
According to question first gas goes from volume V to 3V and after this volume is reduced from 3V to V at constant pressure. In isothermal expansion p-V curve is rectangular hyperbola. Option (d) is correctYou need to login to perform this action.
You will be redirected in
3 sec
