A) zero to +1 and zero to -5
B) zero to -1 and zero to +5
C) zero to -1 and zero to +3
D) zero to +1 and zero to -3
Correct Answer: B
Solution :
When chlorine gas reacts with hot and concentrated \[NaOH\] solution, it disproportionate into chloride \[(C{{l}^{-}})\] and chlorate \[(ClO_{3}^{-})\] ions.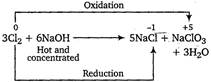 In this process, oxidation number of chlorine changes from 0 to -1 and 0 to +5. Note In disproportionation reactions, the same element undergoes oxidation as well as reduction.
In this process, oxidation number of chlorine changes from 0 to -1 and 0 to +5. Note In disproportionation reactions, the same element undergoes oxidation as well as reduction.
You need to login to perform this action.
You will be redirected in
3 sec
