A) \[\text{ }\!\![\!\!\text{ BC}{{\text{l}}_{\text{3}}}\,\text{and}\,\text{BrC}{{\text{l}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }\]
B) \[\text{ }\!\![\!\!\text{ N}{{\text{H}}_{\text{3}}}\,\text{and}\,\text{NO}_{\text{3}}^{\text{-}}\text{ }\!\!]\!\!\text{ }\]
C) \[\text{ }\!\![\!\!\text{ N}{{\text{F}}_{\text{3}}}\,\text{and}\,\text{B}{{\text{F}}_{\text{3}}}\text{ }\!\!]\!\!\text{ }\]
D) \[\text{ }\!\![\!\!\text{ BF}_{\text{4}}^{\text{-}}\,\text{and}\,\text{NH}_{\text{4}}^{\text{+}}\text{ }\!\!]\!\!\text{ }\]
Correct Answer: C
Solution :
If number of bond pairs and lone pairs are same for the given pairs, they are is structural. \[BC{{l}_{3}}\]and\[BrC{{l}_{3}}\]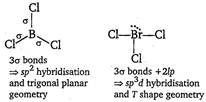 \[N{{H}_{3}}\,\text{and}\,NO_{3}^{-}\]
\[N{{H}_{3}}\,\text{and}\,NO_{3}^{-}\] 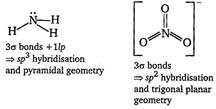 \[N{{F}_{3}}\,\text{and}\,BF_{3}^{{}}\]
\[N{{F}_{3}}\,\text{and}\,BF_{3}^{{}}\] 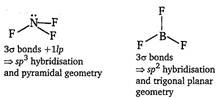 \[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\]
\[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\] 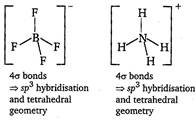 Thus, \[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\]are isostructural.
Thus, \[BF_{4}^{-}\,\text{and}\,NH_{4}^{+}\]are isostructural.
You need to login to perform this action.
You will be redirected in
3 sec
