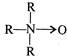
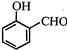
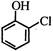
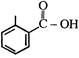
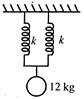
question_answer 1)
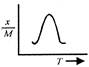
A uniform circular disc of mass 12 kg is held by two identical springs as shown in the figure. When the disc is pressed down slightly and released, it executes SHM with a time period of 2 s. The force constant of each spring is
A)
\[236.6\text{ }N{{m}^{-1}}\]
done
clear
B)
\[118.3\text{ }N{{m}^{-1}}\]
done
clear
C)
\[59.15N{{m}^{-1}}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 2) A cyclist bends, while taking turn, to
A)
reduce friction
done
clear
B)
generate required centrifugal force
done
clear
C)
reduce apparent weight
done
clear
D)
reduce speed
done
clear
View Answer play_arrow
question_answer 3) Consider two unequal masses\[{{m}_{2}}>{{m}_{1}}\]connected by a string which passes over a frictionless and massless pulley. The tension T in the string is
A)
\[T=2\frac{m_{1}^{2}+m_{2}^{2}}{{{m}_{1}}+{{m}_{2}}}g\]
done
clear
B)
\[T=\frac{2{{m}_{1}}{{m}_{2}}}{{{m}_{1}}+{{m}_{2}}}g\]
done
clear
C)
\[T=\frac{2m_{2}^{2}+m_{1}^{2}}{{{m}_{1}}+{{m}_{2}}}g\]
done
clear
D)
\[T=2({{m}_{1}}+{{m}_{2}})g\]
done
clear
View Answer play_arrow
question_answer 4) For motion in a plane with constant acceleration a initial velocity \[{{\overrightarrow{v}}_{0}}\] and final velocity\[\overrightarrow{v}\]after time\[t\]we have
A)
\[\overrightarrow{v}.(\overrightarrow{v}.\overrightarrow{a}t)={{\overrightarrow{v}}_{0}}({{\overrightarrow{v}}_{0}}+\overrightarrow{a}t)\]
done
clear
B)
\[\overrightarrow{v}.{{\overrightarrow{v}}_{0}}={{a}^{2}}{{t}^{2}}\]
done
clear
C)
\[\overrightarrow{v}.{{\overrightarrow{v}}_{0}}=\overrightarrow{a}.{{\overrightarrow{v}}_{0}}t\]
done
clear
D)
\[{{\overrightarrow{v}}_{0}}.{{\overrightarrow{v}}_{0}}=\overrightarrow{a}.{{\overrightarrow{v}}_{0}}\]
done
clear
View Answer play_arrow
question_answer 5) A torch is switched on and dropped on a reflecting surface g/2 meters below. Initial light beam reaches the torch after getting reflected from the polished surface and is reflected back by the torch, and so on. If speed of light is c then total distance covered by the initial beam of light is
A)
g
done
clear
B)
g/2
done
clear
C)
c
done
clear
D)
c/2
done
clear
View Answer play_arrow
question_answer 6) The potential energy function of a particle executing linear simple harmonic motion is given by \[U(x)=\frac{1}{2}k{{x}^{2}},\]where k the force constant of the oscillator is equal to\[0.5\text{ }N{{m}^{-1}}\]. The amplitude of the particle when its total energy is 1 J is equal to
A)
\[2\sqrt{2}m\]
done
clear
B)
2m
done
clear
C)
\[\sqrt{2}m\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 7) A man mass 60 kg records his weight on a weighing machine placed inside a lift. The ratio of the weights of the man recorded when the lift is ascending up with a uniform speed of \[2\text{ }m{{s}^{-1}}\]to when it is descending down with a uniform speed of\[\text{4 }m{{s}^{-1}}\]will be
A)
0.5
done
clear
B)
\[-1\]
done
clear
C)
2
done
clear
D)
none of the above
done
clear
View Answer play_arrow
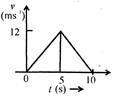
question_answer 8)
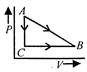
The speed time graph of a particle moving in a fixed direction is as shown in the figure. The distance traversed by the particle between\[t=0\]to\[t=5\text{ }s\]is
A)
24 m
done
clear
B)
30 m
done
clear
C)
36 m
done
clear
D)
40 m
done
clear
View Answer play_arrow
question_answer 9) Units of electric flux are
A)
\[weber/metr{{e}^{2}}\]
done
clear
B)
\[newton-metr{{e}^{2}}/coulomb\]
done
clear
C)
\[joule\times coulomb/metre\]
done
clear
D)
tesla
done
clear
View Answer play_arrow
question_answer 10) The S.I. unit of pole strength is
A)
\[A-{{m}^{2}}\]
done
clear
B)
\[A-m\].
done
clear
C)
\[A-{{m}^{-1}}\]
done
clear
D)
\[A-{{m}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 11) In a streamlined flow if the gravitational head is h, the kinetic and pressure heads are
A)
\[\frac{1}{2}{{v}^{2}}\]and\[\frac{P}{\rho }\]
done
clear
B)
\[\frac{1}{2}\frac{{{v}^{2}}}{g}\]and\[\frac{P}{\rho g}\]
done
clear
C)
\[\frac{1}{2}{{v}^{2}}\]and\[\frac{P}{\rho g}\]
done
clear
D)
\[\frac{1}{2}\frac{{{v}^{2}}}{g}\]and\[\frac{P}{\rho }\]
done
clear
View Answer play_arrow
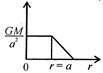
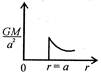
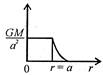
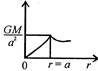
question_answer 12) Which one of the following graphs represents correctly the variation of the intensity of gravitational field\[(I)\]with the distance (r) from the centre of a spherical shell of mass M and radius a
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 13) A quantity X is defined by the equation \[X=3C{{B}^{2}}\]where C is capacitance in farad and B represents magnetic field in tesla. The dimensions of\[X\]are
A)
\[M{{L}^{-2}}\]
done
clear
B)
\[M{{L}^{-2}}{{T}^{-1}}A\]
done
clear
C)
\[M{{L}^{-1}}{{T}^{-2}}{{A}^{2}}\]
done
clear
D)
\[{{L}^{-1}}{{A}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 14) Bernouillis equation for steady, non-viscous, incompressible flow expresses the
A)
conservation of angular momentum
done
clear
B)
conservation of density
done
clear
C)
conservation of momentum
done
clear
D)
conservation of energy
done
clear
View Answer play_arrow
question_answer 15) Velocity of sound in air is\[320\text{ }m{{s}^{-1}}\]. A pipe closed at one end has a length of 1m. (Neglecting ends corrections). The air column in the pipe cannot resonate for sound of frequency
A)
80 Hz
done
clear
B)
240 Hz
done
clear
C)
320 Hz
done
clear
D)
400 Hz
done
clear
View Answer play_arrow
question_answer 16) A particle in S.H.M. is described by the displacement function \[x(t)=A\cos (\omega t+\phi ),\omega =\frac{2\pi }{T}\] If the initial\[(t=0)\]position of the particle is 1 cm, its initial velocity is n\[cm\text{ }{{s}^{-1}}\]and its angular frequency is\[n\text{ }{{s}^{-1}},\]then the amplitude of its motion is
A)
\[\pi \,cm\]
done
clear
B)
\[2\,cm\]
done
clear
C)
\[\sqrt{2}\,cm\]
done
clear
D)
\[1\,cm\]
done
clear
View Answer play_arrow
question_answer 17) Equation\[y={{y}_{m}}\sin (kx+\omega t)\]represents a wave
A)
travelling in\[y-\]direction
done
clear
B)
travelling in negative\[y-\]direction
done
clear
C)
travelling in\[y-\]direction
done
clear
D)
travelling in negative\[y-\]direction
done
clear
View Answer play_arrow
question_answer 18) To double the translational kinetic energy of the molecules of a gas one has to
A)
double the absolute temperature
done
clear
B)
reduce the absolute temperature to half
done
clear
C)
increase the absolute temperature fourfold
done
clear
D)
increase the absolute temperature by a factor of \[\sqrt{2}\]
done
clear
View Answer play_arrow
question_answer 19) The first law of thermodynamics, which accounts for the conservation of energy, is valid
A)
only for reversible processes
done
clear
B)
only for irreversible processes
done
clear
C)
for both of the above two processes
done
clear
D)
for none of the above processes
done
clear
View Answer play_arrow
question_answer 20) A comet orbits the sun in highly elliptical orbit. Which of the following quantities remains unchanged throughout its orbit? (Neglect any mass loss of the comet when it comes very close to the sun)
A)
torque
done
clear
B)
potential energy
done
clear
C)
kinetic energy
done
clear
D)
areal speed
done
clear
View Answer play_arrow
question_answer 21) Two bulbs when connected in parallel to a source take 60 W each. The total power consumed when they are connected in series with the same source is
A)
15 W
done
clear
B)
30 W
done
clear
C)
60 W
done
clear
D)
120 W
done
clear
View Answer play_arrow
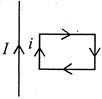
question_answer 22)
A rectangular loop carrying a current\[i\]is situated near a long straight wire, such that the wire is parallel to one of he sides of the loop and is in the plane of the loop. If a steady current\[I\]is established in the wire as shown in the diagram, the loop will
A)
move away from the wire
done
clear
B)
rotate about an axis parallel to wire
done
clear
C)
remain stationary
done
clear
D)
move towards the wire
done
clear
View Answer play_arrow
question_answer 23) In a voltameter the conduction takes place due to
A)
electrons only
done
clear
B)
electrons and holes
done
clear
C)
holes only
done
clear
D)
electrons and ions
done
clear
View Answer play_arrow
question_answer 24) A constant voltage is applied between two ends of a uniform metallic wire. Some heat is developed in it. The heat developed is doubled if
A)
both the length and the radius of the wire are doubled
done
clear
B)
both the length and the radius of the wire are halved
done
clear
C)
the radius of the wire is doubled
done
clear
D)
the length of the wire is doubled
done
clear
View Answer play_arrow
question_answer 25) A magnetic needle placed in a non-uniform magnetic field experiences
A)
a force and a torque
done
clear
B)
a torque but not a force
done
clear
C)
a force but not a torque
done
clear
D)
neither a force not a torque
done
clear
View Answer play_arrow
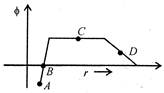
question_answer 26)
In a region of space, the variation of the electric potential\[\phi \]with distance from the origin is shown in the figure. The electric field strength is zero at
A)
Point A
done
clear
B)
Point B
done
clear
C)
Point C
done
clear
D)
Point D
done
clear
View Answer play_arrow
question_answer 27) The instantaneous magnetic flux\[\phi \]in a circuit is \[\phi =4{{t}^{2}}-4t+1\]The total resistance of the circuit is \[10\,\Omega \]. At\[t=\frac{1}{2}s,\]the induced current in the circuit is
A)
0
done
clear
B)
0.2 A
done
clear
C)
0.4 A
done
clear
D)
0.8 A
done
clear
View Answer play_arrow
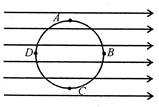
question_answer 28)
A circle of radius R is drawn in a uniform electric field E as shown in the figure\[{{V}_{A}},\]\[{{V}_{B}},{{V}_{C}}\]and\[{{V}_{D}}\]are respectively the potentials of points A, B, C and D at the periphery of the circle
A)
\[{{V}_{A}}={{V}_{C}};{{V}_{B}}={{V}_{D}}\]
done
clear
B)
\[{{V}_{A}}={{V}_{C}};{{V}_{B}}<{{V}_{D}}\]
done
clear
C)
\[{{V}_{A}}>{{V}_{C}};{{V}_{B}}={{V}_{D}}\]
done
clear
D)
\[{{V}_{A}}<{{V}_{C}};{{V}_{B}}={{V}_{D}}\]
done
clear
View Answer play_arrow
question_answer 29) If the distance of separation between two charges is increased, the electrical potential energy of the system will
A)
increase
done
clear
B)
decrease
done
clear
C)
may increase or decrease
done
clear
D)
remain the same
done
clear
View Answer play_arrow
question_answer 30) Let Q represent the charge on a parallel plate capacitor and E the electric field between the plates, then each plate of the capacitor experiences a force of magnitude
A)
zero
done
clear
B)
\[QE\]
done
clear
C)
\[\frac{1}{2}QE\]
done
clear
D)
\[2QE\]
done
clear
View Answer play_arrow
question_answer 31) In the Youngs double slit experiment the separation between the slits is halved and the distance between the slits and the screen is doubled. The fringe width is
A)
halved
done
clear
B)
unchanged
done
clear
C)
doubled
done
clear
D)
quadrupled
done
clear
View Answer play_arrow
question_answer 32) When the light is incident on polarising angle which of the following is completely polarised
A)
reflected light
done
clear
B)
both reflected and refracted light
done
clear
C)
refracted light
done
clear
D)
neither reflected nor refracted light
done
clear
View Answer play_arrow
question_answer 33) Signal from a remote control to the device operated by it travels with the speed of
A)
sound
done
clear
B)
light
done
clear
C)
ultrasonics
done
clear
D)
supersonics
done
clear
View Answer play_arrow
question_answer 34) In spectral radiancy curve for cavity radiation the wavelength of the maximum spectral radiancy
A)
increases with temperature increasing
done
clear
B)
decreases with temperature increasing
done
clear
C)
sometimes increases sometimes decreases with increasing temperature
done
clear
D)
remains unaffected by temperature variation
done
clear
View Answer play_arrow
question_answer 35) For the normal setting of a telescope
A)
only the object is at infinity
done
clear
B)
only the final image is at infinity
done
clear
C)
both the object and the final image are at infinity
done
clear
D)
neither the object nor the final image has to be at infinity
done
clear
View Answer play_arrow
question_answer 36) A beam of light incident on a plane mirror forms a real image on reflection. The incident beam is
A)
parallel
done
clear
B)
convergent
done
clear
C)
divergent
done
clear
D)
any of the above type
done
clear
View Answer play_arrow
question_answer 37) If\[{{\varepsilon }_{0}}\]and\[{{\mu }_{0}}\]are respectively, the electric permittivity and magnetic permeability of free space,\[\varepsilon \]and\[\mu \]are the corresponding quantities in a medium the refractive index of the medium is
A)
\[\sqrt{\frac{\mu \varepsilon }{{{\mu }_{0}}{{\varepsilon }_{0}}}}\]
done
clear
B)
\[\sqrt{\frac{{{\mu }_{0}}{{\varepsilon }_{0}}}{{{\varepsilon }_{0}}}}\]
done
clear
C)
\[\frac{\mu \varepsilon }{{{\mu }_{0}}{{\varepsilon }_{0}}}\]
done
clear
D)
\[{{\left( \frac{\mu \varepsilon }{{{\mu }_{0}}{{\varepsilon }_{0}}} \right)}^{2}}\]
done
clear
View Answer play_arrow
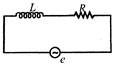
question_answer 38)
In the given circuit, the rms value of e is 5V and the rms value of votage drop across L is 3 V. The rms value of the voltage across R will be
A)
2V
done
clear
B)
3 V
done
clear
C)
4 V
done
clear
D)
0 V
done
clear
View Answer play_arrow
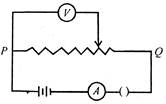
question_answer 39)
The figure shows the connections of a rheostat with an external battery. A is an ammeter and is a voltmeter. The sliding contact R starts from point P and moves towards the end Q. Which of the following statements is correct?
A)
both ammeter and voltmeter show constant reading
done
clear
B)
the reading of ammeter remains constant but that of voltmeter increases
done
clear
C)
the reading of ammeter remains constant but that of voltmeter decreases
done
clear
D)
both ammeter and voltmeter reading increases
done
clear
View Answer play_arrow
question_answer 40) A conducting wire is drawn to double its length. Final resistivity of the material will be
A)
double of the original one
done
clear
B)
half of the original one.
done
clear
C)
one-fourth of the original one
done
clear
D)
same as original one
done
clear
View Answer play_arrow
question_answer 41) A\[p-\]type semiconductor is
A)
positively charged
done
clear
B)
uncharged
done
clear
C)
negatively charged
done
clear
D)
none of the above
done
clear
View Answer play_arrow
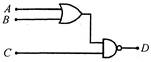
question_answer 42)
For the given combination of gates, if the logic states of input A, B, C are as follows\[A=B=C=0\]and\[A=B=1,C=0\]then the logic states of output\[D\]are
A)
0, 0
done
clear
B)
0, 1
done
clear
C)
1, 0
done
clear
D)
1, 1
done
clear
View Answer play_arrow
question_answer 43) A material with overlapping conduction and valence bands will be
A)
an insulator
done
clear
B)
a semiconductor
done
clear
C)
a metal
done
clear
D)
a superconductor
done
clear
View Answer play_arrow
question_answer 44) Assuming that the junction diode is ideal, in the circuit shown here, the current through the diode is
A)
zero
done
clear
B)
1 mA
done
clear
C)
10 mA
done
clear
D)
30 Ma
done
clear
View Answer play_arrow
question_answer 45) When a beta\[(\beta )\]negative particle is emitted from a radioactive nucleus, the ratio of the number of neutrons to the number of protons in the resulting nucleus
A)
decreases
done
clear
B)
remains same
done
clear
C)
increases
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 46) An electron starts from rest in an electric field and acquires a speed u in reaching a point A. The potential difference between the starting point and the point A is\[{{V}_{A}}\]
A)
\[u\propto V_{A}^{2}\]
done
clear
B)
\[u\propto {{V}_{A}}\]
done
clear
C)
\[u\propto \sqrt{{{V}_{A}}}\]
done
clear
D)
\[u\propto V_{A}^{2/3}\]
done
clear
View Answer play_arrow
question_answer 47) The binding energy per nucleon for deuteron\[(_{1}^{2}H)\]and helium\[(_{2}^{4}He)\]are 1.1 MeV and 7.0 MeV and 7.0 MeV respectively. The energy released when two deuterons fuse to form a helium nucleus is
A)
2.2 MeV
done
clear
B)
28 MeV
done
clear
C)
23.6 V
done
clear
D)
30.2 MeV
done
clear
View Answer play_arrow
question_answer 48) The de Broglie wavelength of a particle of mass 1 mg moving with a velocity\[1\text{ }m{{s}^{-1}}\]will be
A)
\[{{10}^{3}}h\] metre
done
clear
B)
\[{{10}^{5}}h\]metre
done
clear
C)
\[{{10}^{-3}}h\]metre
done
clear
D)
\[{{10}^{-5}}h\]metre
done
clear
View Answer play_arrow
question_answer 49) Sodium surface is subjected to ultraviolet and infrared radiations separately and the stopping potential of photoelectrons determined. Then the stopping potential
A)
is equal in both cases
done
clear
B)
more when ultraviolet is used
done
clear
C)
more when infrared used
done
clear
D)
may be more or less for ultraviolet depending upon its intensity as compared to the intensity of infrared light
done
clear
View Answer play_arrow
question_answer 50) An\[\alpha \]particle and a proton having same momentum enter into a region of uniform magnetic field and move in circular paths. The ratio of the radii of curvature of their paths,\[\frac{{{r}_{\alpha }}}{{{r}_{p}}}\], in the field is
A)
\[\frac{1}{2}\]
done
clear
B)
\[\frac{1}{4}\]
done
clear
C)
1
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 51) Which of the following is not a transition metal?
A)
\[Cr\]
done
clear
B)
\[Mn\]
done
clear
C)
\[Sn\]
done
clear
D)
\[Cu~\]
done
clear
View Answer play_arrow
question_answer 52) Copper reacts with hot cone.\[{{H}_{2}}S{{O}_{4}}\]to give
A)
\[S{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}S\]
done
clear
C)
\[CuS{{O}_{3}}\]
done
clear
D)
\[C{{u}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 53) What is the approximate percentage of\[{{H}_{2}}{{O}_{2}}\] in a sample labelled as 10 vol?
A)
10%
done
clear
B)
9%
done
clear
C)
3%
done
clear
D)
1%
done
clear
View Answer play_arrow
question_answer 54) Addition of\[HgC{{l}_{2}}\]to\[SnC{{l}_{2}}\]gives a brown black colour which is due to the
A)
formation of amalgam
done
clear
B)
oxidation of tin
done
clear
C)
oxidation of mercury
done
clear
D)
reduction of mercury
done
clear
View Answer play_arrow
question_answer 55) Name the compound\[{{O}_{2}}Pt{{F}_{6}}\].
A)
oxygenyl fluoroplatinate (IV)
done
clear
B)
dioxygenyl fluoroplatinate (V)
done
clear
C)
dioxygenyl hexafluoroplatinate (IV)
done
clear
D)
dioxygenyl hexafluoroplatinate (V)
done
clear
View Answer play_arrow
question_answer 56) A gas has a volume of 15 ml at 300 K and 740 mm of Hg. Find the temperature if volume becomes 10 ml at 760 mm pressure of Hg.
A)
205 K
done
clear
B)
209 K
done
clear
C)
275 K
done
clear
D)
\[-200\text{ }K\]
done
clear
View Answer play_arrow
question_answer 57) Which one of the following is the strongest acid?
A)
\[Cl{{O}_{3}}(OH)\]
done
clear
B)
\[Cl{{O}_{2}}(OH)\]
done
clear
C)
\[HCl\]
done
clear
D)
\[HN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 58) Phosphorus on reaction with cone\[HN{{O}_{3}}\]yields
A)
\[HP{{O}_{3}}\]
done
clear
B)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
C)
\[{{N}_{2}}O\]
done
clear
D)
\[{{N}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 59) Malachite is a mineral of
A)
\[Mg\]
done
clear
B)
\[Cu\]
done
clear
C)
\[Al\]
done
clear
D)
\[Fe\]
done
clear
View Answer play_arrow
question_answer 60) Which is the correct decreasing order of bond angle in the following series?
A)
\[N{{H}_{3}}>{{H}_{2}}O>{{H}_{2}}S\]
done
clear
B)
\[{{H}_{2}}S>N{{H}_{3}}>{{H}_{2}}O\]
done
clear
C)
\[{{H}_{2}}S>{{H}_{2}}O>N{{H}_{3}}\]
done
clear
D)
\[{{H}_{2}}O>N{{H}_{3}}>{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 61) Which profile is a true representation of chemosorption?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 62) A process for converting a precipitate into a colloidal solution is known as
A)
coagulation
done
clear
B)
peptization
done
clear
C)
dialysis
done
clear
D)
ozonolysis
done
clear
View Answer play_arrow
question_answer 63) When river water meets sea water delta formation takes place. This is due to the phenomenon of
A)
electrophoresis
done
clear
B)
dialysis
done
clear
C)
coagulation
done
clear
D)
adsorption
done
clear
View Answer play_arrow
question_answer 64) 1.5 g of a substance when dissolved in 60 g water lowered the freezing point by\[0.136{}^\circ C\]. Calculate the molecular weight of the substance when depression constant of water is 1.86.
A)
329.1
done
clear
B)
439.5
done
clear
C)
341.9
done
clear
D)
335.9
done
clear
View Answer play_arrow
question_answer 65)
Consider the modes of transformations of a gas from state A to state B as shown in the following P-V diagram Which one of the following is true?
A)
\[\Delta H=q\]along\[A\to C\]
done
clear
B)
\[\Delta S\]is same along both\[A\to B\]and \[A\to C\to B\]
done
clear
C)
\[w\]is same along both\[A\to B\]and\[A\to C\to B\]
done
clear
D)
\[w>0\]along both\[A\to B\]and\[A\to C\]
done
clear
View Answer play_arrow
question_answer 66) Sulphur tetrafluoride molecule is
A)
square planar
done
clear
B)
octahedral
done
clear
C)
tetrahedral
done
clear
D)
trigonal bipyramidal with a lone pair in the equatorial position
done
clear
View Answer play_arrow
question_answer 67) The types of bonds present in\[CuS{{O}_{4}}.5{{H}_{2}}O\] are only
A)
electrovalent and covalent
done
clear
B)
electrovalent and coordinate-covalent
done
clear
C)
electrovalent, covalent and coordinate covalent
done
clear
D)
electrovalent, covalent and hydrogen
done
clear
View Answer play_arrow
question_answer 68) The ionisation potential for hydrogen atom is 13.6 eV, the ionisation potential for the\[H{{e}^{+}}\]is
A)
54.4 eV
done
clear
B)
6.8 eV
done
clear
C)
13.6eV
done
clear
D)
24.5 eV
done
clear
View Answer play_arrow
question_answer 69) Molecular shape of\[P{{F}_{5}}\]is
A)
square planar
done
clear
B)
trigonal bipyramidal
done
clear
C)
tetrahedral three-dimensional
done
clear
D)
octahedral three-dimensional
done
clear
View Answer play_arrow
question_answer 70) Which is not formed in a reaction of potassium permanganate with\[{{H}_{2}}S{{O}_{4}}\]?
A)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[MnS{{O}_{4}}\]
done
clear
C)
\[Mn{{O}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 71) Consider the following half cell reactions: \[C{{u}^{2+}}+2{{e}^{-}}=Cu\] \[E_{C{{u}^{2+}}/Cu}^{o}=0.34\,V\] \[A{{g}^{+}}+{{e}^{-}}=Ag\] \[E_{A{{g}^{+}}/Ag}^{o}=0.80\,V\] Which one of the following is true?
A)
copper can displace silver from\[AgN{{O}_{3}}\]solution
done
clear
B)
silver can displace copper from\[Cu{{(N{{O}_{3}})}_{2}}\]solution
done
clear
C)
silver is more reactive than copper
done
clear
D)
\[C{{u}^{2+}}/Cu\]and\[A{{g}^{+}}/Ag\]with positive\[E{}^\circ \]values cannot be coupled to form a Galvanic cell
done
clear
View Answer play_arrow
question_answer 72) The maximum work which a system can perform at constant temperature and pressure equals
A)
\[q-\Delta E\]
done
clear
B)
\[\Delta H-\Delta E\]
done
clear
C)
\[P\Delta V-\Delta G\]
done
clear
D)
\[(-\Delta G)\]
done
clear
View Answer play_arrow
question_answer 73) The equation\[\Delta G=\Delta H-T\Delta S\]is for
A)
open system
done
clear
B)
closed system
done
clear
C)
isolated system
done
clear
D)
for all the above
done
clear
View Answer play_arrow
question_answer 74) If the melting point of ice is\[0{}^\circ C\]and latent heat of fusion is\[5.46\text{ }KJ\text{ }mo{{l}^{-1}},\] the entropy change for the conversion of 18 g of ice to water at 1 atm is
A)
zero
done
clear
B)
\[20\text{ }J{{K}^{-1}}\]
done
clear
C)
\[50\text{ }J{{K}^{-1}}\]
done
clear
D)
\[\text{360 }J{{K}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 75) During the crystallization of a solid from the aqueous solution, the following statement is correct\[(\Delta H=-ve)\]
A)
\[\Delta H=T\Delta S\]
done
clear
B)
\[\Delta H>T\Delta S\]
done
clear
C)
\[\Delta H<T\Delta S\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 76) The\[p{{K}_{a}}\]of acetyl salicylic acid (aspirin) is 3.5. The pH of gastric juice in human stomach is around 2 and the pH in small intestine is around 8. Aspirin will be
A)
unionized in stomach as well as in intestine
done
clear
B)
ionized in stomach as well as in intestine
done
clear
C)
unionized in stomach but ionized in intestine
done
clear
D)
ionized in stomach but unionized in intestine
done
clear
View Answer play_arrow
question_answer 77) In the reaction\[A+B\rightleftharpoons 2C,\]the initial concentrations of A and B are 1.0 and 2.0 mol \[li{{t}^{-1}}\]respectively. If at equilibrium the concentration of C is 0.4 mol\[li{{t}^{-1}}\], the value of equilibrium constant is
A)
0.028
done
clear
B)
0.111
done
clear
C)
0.166
done
clear
D)
0.200
done
clear
View Answer play_arrow
question_answer 78) If a 0.00001 molar solution of\[HCl\]is diluted thousand folds the pH of the resulting solution will be
A)
5
done
clear
B)
7
done
clear
C)
8
done
clear
D)
6.98
done
clear
View Answer play_arrow
question_answer 79) The conjugate acid of\[{{(C{{H}_{3}})}_{2}}NH\]is
A)
\[{{(C{{H}_{3}})}_{2}}N-COOH\]
done
clear
B)
\[{{(C{{H}_{3}})}_{2}}{{N}^{-}}\]
done
clear
C)
\[{{(C{{H}_{3}})}_{2}}{{N}^{+}}\]
done
clear
D)
\[{{(C{{H}_{3}})}_{2}}NH_{2}^{+}\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following solution has the highest osmotic pressure?
A)
\[0.1\text{ }M\text{ }HCl\]
done
clear
B)
0.1 M urea
done
clear
C)
\[0.1\,M\,BaC{{l}_{2}}\]
done
clear
D)
0.1 M glucose
done
clear
View Answer play_arrow
question_answer 81) Which one of the following is a trihydric alcohol containing only secondary hydroxyl groups?
A)
\[\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,\]
done
clear
B)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
C)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ HO \end{smallmatrix}}{\mathop{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-OH\]
done
clear
D)
\[\underset{\begin{smallmatrix} | \\ HO \end{smallmatrix}}{\mathop{C{{H}_{2}}}}\,-\underset{\begin{smallmatrix} | \\ HO \end{smallmatrix}}{\mathop{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,}}\,-\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 82) Which one of the following compounds is likely to be optically active?
A)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}-C{{H}_{3}}\]
done
clear
B)
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{3}}\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 83) Carbon carbon length is the shortest in
A)
ethane
done
clear
B)
ethene
done
clear
C)
ethyne
done
clear
D)
benzene
done
clear
View Answer play_arrow
question_answer 84) Nitrogen in an organic compound can be estimated by
A)
Kjeldahl method
done
clear
B)
Carius method
done
clear
C)
Victor Meyers method
done
clear
D)
Lassaignes method
done
clear
View Answer play_arrow
question_answer 85) Which one has a chiral centre?
A)
2-methylpentane
done
clear
B)
3-methylpentane
done
clear
C)
2, 3-dimethylpentane
done
clear
D)
2, 3, 4-trimethylpentane
done
clear
View Answer play_arrow
question_answer 86) How much copper is supposed to be deposited when a current of 0.75 amperes passes through a copper sulphate solution for 25 minutes?
A)
0.34 g
done
clear
B)
0.33 g
done
clear
C)
0.35 g
done
clear
D)
( d) 0.37 g
done
clear
View Answer play_arrow
question_answer 87) Which of the following metals will not react with solution of\[CuS{{O}_{4}}\]?
A)
\[Fe\]
done
clear
B)
\[Zn\]
done
clear
C)
\[Mg\]
done
clear
D)
\[Ag\]
done
clear
View Answer play_arrow
question_answer 88) The decay from\[_{92}^{235}U\]to\[_{82}^{211}Pb\]involves the emission of
A)
\[2\alpha \] and\[6\beta \]particles
done
clear
B)
\[5\alpha \] and\[6\beta \]particles
done
clear
C)
\[6\alpha \] and\[2\beta \]particles
done
clear
D)
\[10\alpha \] and\[6\beta \]particles
done
clear
View Answer play_arrow
question_answer 89) In the reaction\[_{1}^{2}H+_{1}^{2}H\to _{2}^{4}He,5.5\times {{10}^{8}}\]K cal/mole of energy is produced. Calculate the energy per gram of He
A)
\[5.50\times {{10}^{8}}K.cal\]
done
clear
B)
\[1.10\times {{10}^{8}}K.cal\]
done
clear
C)
\[2.75\times {{10}^{8}}K.cal\]
done
clear
D)
\[1.37\times {{10}^{8}}K.cal\]
done
clear
View Answer play_arrow
question_answer 90) The missing particle in the radioactive decay is\[_{15}^{30}P\xrightarrow[{}]{{}}\,_{14}^{30}Si+?\]
A)
electron
done
clear
B)
hydrogen atom
done
clear
C)
positron
done
clear
D)
neutron
done
clear
View Answer play_arrow
question_answer 91) \[R\,C{{H}_{2}}\,C{{H}_{2}}\,CN\]can be easily obtained by which of the following reaction?
A)
\[RCH=C{{H}_{2}}+HCN\]
done
clear
B)
\[R\,C{{H}_{2}}C{{H}_{3}}+HCN\]
done
clear
C)
\[R\,C{{H}_{2}}C{{H}_{2}}-Br+KCN\]
done
clear
D)
\[R\,C{{H}_{2}}-CON{{H}_{2}}+{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 92) Which one of the following chemicals can be used in the preparation of paracetamol?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) Ethyne can be oxidised to oxalic acid by using
A)
chromic acid
done
clear
B)
alkaline\[KMn{{O}_{4}}\]
done
clear
C)
hypochlorous acid
done
clear
D)
all reagents
done
clear
View Answer play_arrow
question_answer 94) Enzymes are essential as bio-catalysts. They function well in
A)
aqueous medium, temp\[\simeq 30-35{}^\circ C;pH\simeq 7\]
done
clear
B)
organic medium (solvent)
done
clear
C)
aqueous medium in extreme pH conditions
done
clear
D)
none of them
done
clear
View Answer play_arrow
question_answer 95) Which compound/set of compounds is used in the manufacture of Nylon 6?
A)
\[HOOC(C{{H}_{2}})COOH+N{{H}_{2}}{{(C{{H}_{2}})}_{6}}N{{H}_{2}}\]
done
clear
B)
done
clear
C)
done
clear
D)
\[C{{H}_{2}}=CH-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 96)
In the reaction
A)
\[NO_{2}^{-}\]
done
clear
B)
\[NO_{2}^{+}\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 97) Identify the compound which has a nitro group in it
A)
\[C{{H}_{3}}-O-N=O\]
done
clear
B)
done
clear
C)
\[C{{H}_{3}}-N=O\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 98) Which reagent is effective in direct conversion of a carboxylic group with a\[1{}^\circ -\] alcoholic group? \[(-COOH\to -C{{H}_{2}}OH)\]
A)
Na-Ethanol
done
clear
B)
\[NaB{{H}_{4}}\]
done
clear
C)
Catalytic hydrogenation
done
clear
D)
\[LiAl{{H}_{4}}\]
done
clear
View Answer play_arrow
question_answer 99) For the preparation of t-butyl methyl ether which one of the following methods should be recommended?
A)
\[C{{H}_{3}}Br+\overset{+}{\mathop{Na}}\,\overline{O}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\]
done
clear
B)
\[C{{H}_{3}}\overline{O}\overset{+}{\mathop{Na}}\,+C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-Br\]
done
clear
C)
\[C{{H}_{3}}OH+HO-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{3}}\xrightarrow[{}]{conc/{{H}_{2}}S{{O}_{4}}}\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-Br+C{{H}_{3}}OH\xrightarrow[{}]{\overset{-}{\mathop{HO}}\,\overset{+}{\mathop{Na}}\,}\]
done
clear
View Answer play_arrow
question_answer 100) Reaction of phenol with\[CC{{l}_{4}}\]and\[NaOH\] followed by hydrolysis is likely to give
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 101) Municipal waste, containing human and animal excreta, food residues, detergents etc. and rich in bacteria and organic substances, is called
A)
sewage
done
clear
B)
sewer
done
clear
C)
sewerage
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 102) BOD stand for
A)
bacterial overgrowth database
done
clear
B)
biological oxygen demand
done
clear
C)
biological and organic diversity
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 103) The pulling strength of community waste water is usually characterized by its
A)
BOD
done
clear
B)
CFC
done
clear
C)
MIC
done
clear
D)
ABS.
done
clear
View Answer play_arrow
question_answer 104) When the pollutant flow is conveyed in well defined channels as municipal or industrial waste it is called
A)
diffuse source
done
clear
B)
non-point source
done
clear
C)
point source
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 105) Water comes polluted due to the presence or addition of
A)
inorganic substances
done
clear
B)
organic substances
done
clear
C)
biological agents
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 106) Photochemical combination of hydrocarbons and nitrogen oxides emitted by automobiles produces health threatening
A)
acid rains
done
clear
B)
CFC
done
clear
C)
fly ash
done
clear
D)
smog.
done
clear
View Answer play_arrow
question_answer 107) The removal of lead and its products from petrol used in automobiles is necessary because lead
A)
causes cancer of skin
done
clear
B)
impairs respiration
done
clear
C)
hampers haemoglobin formation
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 108) Large quantities of carbon monoxide, produced by gas heaters, charcoal stoves, coal mines etc., usually prove fatal as the pollutant
A)
is carcinogenic
done
clear
B)
impairs respiration
done
clear
C)
is combustible
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 109) Air pollution is caused by excess of
A)
dinitrogen
done
clear
B)
hydrogen
done
clear
C)
water vapour
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 110) The indiscriminate use of chemical fertilizers and pesticides may cause pollution of
A)
air
done
clear
B)
soil
done
clear
C)
water
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 111) Spadix is an inflorescence found only in
A)
monocots
done
clear
B)
dicots
done
clear
C)
poaceae
done
clear
D)
asteraceae.
done
clear
View Answer play_arrow
question_answer 112) The agency responsible for seed dispersal in cotton, poppy and orchids is
A)
bird
done
clear
B)
water
done
clear
C)
man
done
clear
D)
wind.
done
clear
View Answer play_arrow
question_answer 113) Among the following sets of fruits, those that belong to the same category, from the point of view of classification, are
A)
coconut, chestnut and cashewnut
done
clear
B)
coconut, mango and almond
done
clear
C)
coconut, butternut and chestnut
done
clear
D)
coconut, orange and tomato.
done
clear
View Answer play_arrow
question_answer 114) In general, the feature common to the flowers of spadix, cyathium and hypanthodium is that they are
A)
monandrous
done
clear
B)
synandrous
done
clear
C)
diclinous
done
clear
D)
achlamydeous.
done
clear
View Answer play_arrow
question_answer 115) Floral diagram fails to indicate
A)
epiphylly and epipetaly
done
clear
B)
aestivation and placentation
done
clear
C)
position of ovary on the thalamus
done
clear
D)
cohesion of carpels and stamens.
done
clear
View Answer play_arrow
question_answer 116) Porous dehiscence of capsular fruits is seen in
A)
okra
done
clear
B)
poppy
done
clear
C)
cotton
done
clear
D)
datura
done
clear
View Answer play_arrow
question_answer 117) Explosive fruits are a characteristic of
A)
balsam
done
clear
B)
poppy
done
clear
C)
castor
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 118) Cladode is a characteristic morphological feature of
A)
Asparagus and Riiscus
done
clear
B)
Casuarina and Opuntia
done
clear
C)
Cladophora and Cactus
done
clear
D)
Citrus and Euphorbia
done
clear
View Answer play_arrow
question_answer 119) Nuclear reactor malfunction in any country is a cause of concern for
A)
the reactors employees and neighbours
done
clear
B)
the entire human population
done
clear
C)
some enlightened persons world-wide
done
clear
D)
some developed and developing countries.
done
clear
View Answer play_arrow
question_answer 120) Eutrophication of water bodies is associated with water
A)
hole
done
clear
B)
hyacinth
done
clear
C)
lily
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 121) Cork cambium is also called
A)
phellem
done
clear
B)
phellogen
done
clear
C)
phelloderm
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 122) The division of an individual vascular cambial cell results in the formation of
A)
two xylem mother cells
done
clear
B)
two phloem mother cells
done
clear
C)
one xylem and one phloem mother cell
done
clear
D)
one xylem or one phloem mother cell and one cambium cell.
done
clear
View Answer play_arrow
question_answer 123) The vascular bundles of dicot roots are
A)
endarch and polyarch
done
clear
B)
exarch and polyarch
done
clear
C)
exarch and radial
done
clear
D)
endarch and collateral.
done
clear
View Answer play_arrow
question_answer 124) The difference between alburnum and duramen is the presence in the former of
A)
companion cell
done
clear
B)
living cells
done
clear
C)
raw cells
done
clear
D)
lignified cells.
done
clear
View Answer play_arrow
question_answer 125) Fascicular, interfascicuiar and extra stelar cambium together constitute
A)
lateral meristems
done
clear
B)
apical meristems
done
clear
C)
intercalary meristems
done
clear
D)
ground meristems.
done
clear
View Answer play_arrow
question_answer 126) A monocot root is characterized by vascular bundles that are closed as well as
A)
endarch, collateral and tri-to pentarch
done
clear
B)
exarch, polyarch and radial
done
clear
C)
endarch, polyarch and radial
done
clear
D)
collateral, exarch and polyarch.
done
clear
View Answer play_arrow
question_answer 127) The protective tissue in the plant body consists of
A)
xylem, phloem and cambium
done
clear
B)
epidermis, cork and bark
done
clear
C)
sclerenchyma, prosenchyma and collenchyma
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 128) The difference between shoot and root apex lies in lateral appendages being
A)
present in the former
done
clear
B)
absent in the former
done
clear
C)
present in the latter
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 129) The most highly evolved and perfect type of inflorescence is
A)
hypanthodium
done
clear
B)
spadix
done
clear
C)
capitulum
done
clear
D)
polychasial cyme.
done
clear
View Answer play_arrow
question_answer 130) The flowers in a cyathium resemble the ray florets of sunflower in being
A)
sessile
done
clear
B)
imperfect
done
clear
C)
neuter
done
clear
D)
bisexual.
done
clear
View Answer play_arrow
question_answer 131) Microsporogenesis is the
A)
development of megaspore
done
clear
B)
development of pollen grain
done
clear
C)
development of male gametophyte
done
clear
D)
development of female gametophyte.
done
clear
View Answer play_arrow
question_answer 132) In a mature fertilized angiosperm ovule\[n,2n\]and 3n condition is found respectively in
A)
antipodals, synergids and integuments
done
clear
B)
egg, antipodals and nucellus
done
clear
C)
antipodals, egg and endosperm
done
clear
D)
endosperm, nucellus and egg.
done
clear
View Answer play_arrow
question_answer 133) Pollination brought about by different flowers of the same individual plant is called
A)
allogamy
done
clear
B)
autogamy
done
clear
C)
cleistogamy
done
clear
D)
geitonogamy.
done
clear
View Answer play_arrow
question_answer 134) The highly resistant biological material of which the exine of a pollen grain is made up is called
A)
cutin
done
clear
B)
lignin
done
clear
C)
suberin
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 135) The functional megaspore develops after three successive mitotic division into
A)
embryo
done
clear
B)
embryo sac
done
clear
C)
ovule
done
clear
D)
zygote.
done
clear
View Answer play_arrow
question_answer 136) Most plant cells are surrounded by a cell wall. There are some exceptions, for example
A)
bacteria
done
clear
B)
gametes
done
clear
C)
stem hairs
done
clear
D)
root hairs.
done
clear
View Answer play_arrow
question_answer 137) The similarity between palisade and spongy parenchyma of dorsiventral leaf lies in their
A)
arrangement
done
clear
B)
function
done
clear
C)
shape
done
clear
D)
size.
done
clear
View Answer play_arrow
question_answer 138) Some common commercial fibres, like jute and flax, are obtained from
A)
collenchyma
done
clear
B)
chlorenchyma
done
clear
C)
sclerenchyma
done
clear
D)
sclereids.
done
clear
View Answer play_arrow
question_answer 139) Collenchyma resembles parenchyma in being
A)
potentially meristematic
done
clear
B)
partially cutinised
done
clear
C)
provided with air spaces
done
clear
D)
devoid of chloroplasts.
done
clear
View Answer play_arrow
question_answer 140) The nature of sieve elements in the phloem are
A)
dead
done
clear
B)
enucleate
done
clear
C)
thick-walled
done
clear
D)
living but non-functional.
done
clear
View Answer play_arrow
question_answer 141) Maintenance of ecosystems depends upon
A)
food-chains and webs
done
clear
B)
energy flow
done
clear
C)
materials cycles
done
clear
D)
viruses.
done
clear
View Answer play_arrow
question_answer 142) Metabolism, replication and homeostasis are the main characteristics of
A)
eukaryotes
done
clear
B)
prokaryotes
done
clear
C)
organisms
done
clear
D)
viruses.
done
clear
View Answer play_arrow
question_answer 143) The first organisms to appear on earth were
A)
photoautotrophs
done
clear
B)
chemoautotrophs
done
clear
C)
chemoheterotrophs
done
clear
D)
coacervates.
done
clear
View Answer play_arrow
question_answer 144) From the point of view of early chemical evolution that preceded the origin of life on earth, the most important simple organic molecules formed were
A)
sugars and amino-acids
done
clear
B)
glycerol and fatty acids
done
clear
C)
purines and pyrimidines
done
clear
D)
all of the above.
done
clear
View Answer play_arrow
question_answer 145) Whereas todays atmosphere contains gases like\[C{{O}_{2}}\]and\[{{H}_{2}}O\]vapour, but at the time of the origin of life was made up mainly of
A)
\[N{{H}_{3}},C{{H}_{4}}\]and\[{{H}_{2}}O\]vapour
done
clear
B)
\[NO,N{{O}_{2}}\]and\[C{{O}_{2}}\]
done
clear
C)
\[N{{H}_{3}},N{{O}_{2}}\]and\[{{H}_{2}}O\]vapour
done
clear
D)
\[C{{H}_{4}},NO\]and\[C{{O}_{2}}\].
done
clear
View Answer play_arrow
question_answer 146) The long gap of 300 million years between the origin of earth and of life on it was due to lack of
A)
oxygen
done
clear
B)
water
done
clear
C)
DNA
done
clear
D)
suitable temperature.
done
clear
View Answer play_arrow
question_answer 147) Whereas the earth is believed to be 4,500 million years old, life on this planet is estimated to have originated not earlier than
A)
4,200 million years ago
done
clear
B)
3,500 million years ago
done
clear
C)
1, 600 million years ago
done
clear
D)
459 million years ago.
done
clear
View Answer play_arrow
question_answer 148) Pollination by bats is called
A)
anemophily
done
clear
B)
hydrophily
done
clear
C)
ornithophily
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 149) In nature, allogamy is met within
A)
unisexual flowers only
done
clear
B)
neuter flowers only
done
clear
C)
underground flowers only
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 150) Anther culture may be used as a technique for producing haploid plants. However, some diploid plants may also be obtained in the process due to
A)
fusion of male and vegetative cells
done
clear
B)
development of exine and entine
done
clear
C)
development of another wall cells
done
clear
D)
fusion of male cells.
done
clear
View Answer play_arrow
question_answer 151) The theory of continental drift was originally proposed by
A)
Alfred Wegener
done
clear
B)
Robert I Bowman
done
clear
C)
Charles Darwin
done
clear
D)
Alfred Bailey.
done
clear
View Answer play_arrow
question_answer 152) Splint bone is a vestigial structure in
A)
man
done
clear
B)
whale
done
clear
C)
horse
done
clear
D)
Rhea.
done
clear
View Answer play_arrow
question_answer 153) The primitive mammals originated during
A)
triassic period
done
clear
B)
Jurassic period
done
clear
C)
cretaceous period
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 154) Author of the book Historia Generalis Plantarum is
A)
Caroleus Linnaeus
done
clear
B)
George Bentham
done
clear
C)
John Ray
done
clear
D)
Dalton Hooker.
done
clear
View Answer play_arrow
question_answer 155) Most petroleum bearing regions shows the presence of fossil states of
A)
Tetrahymena
done
clear
B)
Radiolaria
done
clear
C)
Paramecium
done
clear
D)
Trichonympha.
done
clear
View Answer play_arrow
question_answer 156) Sea cucumbers belong to class
A)
echinoidea
done
clear
B)
holothuroidea
done
clear
C)
ophiuroidea
done
clear
D)
asteroidea.
done
clear
View Answer play_arrow
question_answer 157) Skull is dicondylic in
A)
reptilia
done
clear
B)
aves
done
clear
C)
mammalia
done
clear
D)
both [b] and [c].
done
clear
View Answer play_arrow
question_answer 158) Botryoidal tissue is found in
A)
hirudina
done
clear
B)
polychaeta
done
clear
C)
oligochaeta
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 159) The larva of Petromyzon is known as
A)
redia
done
clear
B)
spat
done
clear
C)
ammocoete
done
clear
D)
tornaria.
done
clear
View Answer play_arrow
question_answer 160) The theory of chemical evolution of life was experimentally proved by
A)
Miller
done
clear
B)
Haldane
done
clear
C)
Redi
done
clear
D)
Spallanzani.
done
clear
View Answer play_arrow
question_answer 161) In presence of vasopressin, the greatest fraction of filtered water is absorbed in the
A)
loop of Henie
done
clear
B)
proximal tubule
done
clear
C)
distal tubule
done
clear
D)
collecting duct.
done
clear
View Answer play_arrow
question_answer 162) Which of the following is not primarily a function of blood plasma?
A)
transport of\[{{O}_{2}}\]
done
clear
B)
transport of hormones
done
clear
C)
transport of antibodies
done
clear
D)
maintenance of RBC size.
done
clear
View Answer play_arrow
question_answer 163) In an ECG, the QRS stands for
A)
arterial depolarization
done
clear
B)
ventricular depolarization
done
clear
C)
arterial repolarization
done
clear
D)
ventricular repolarization.
done
clear
View Answer play_arrow
question_answer 164) Thrombin indirectly deactivates factor VIII and V via
A)
antithrombin II
done
clear
B)
protein C
done
clear
C)
tissue factor, pathway inhibtor
done
clear
D)
plasmin.
done
clear
View Answer play_arrow
question_answer 165) Which one of the following is an example of a conjugated protein?
A)
casein
done
clear
B)
albumin
done
clear
C)
globulin
done
clear
D)
globin.
done
clear
View Answer play_arrow
question_answer 166) Which of the following minerals needed for the activity of enzymes required for the synthesis of oligosaccharides and glycoproteins?
A)
\[Mn\]
done
clear
B)
\[Mg\]
done
clear
C)
\[Cu\]
done
clear
D)
\[Mo\]
done
clear
View Answer play_arrow
question_answer 167) The major fibrous proteins that provide external protection to vertebrates are
A)
collagen
done
clear
B)
elastin
done
clear
C)
\[\alpha -\]keratins
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 168) Desmosomes are
A)
a special type of chromosomes
done
clear
B)
a cell organelle
done
clear
C)
cell junction of connective tissue
done
clear
D)
cell junction of epithelial tissue.
done
clear
View Answer play_arrow
question_answer 169) The secretory epithelium of sebaceous glands is
A)
cuboidal
done
clear
B)
stratified squamous
done
clear
C)
columnar
done
clear
D)
stratified columnar.
done
clear
View Answer play_arrow
question_answer 170) Argyrophilic fibre is another name for
A)
collagen fibre
done
clear
B)
reticular fibre
done
clear
C)
elastic fibre
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 171) Diagrammatic representation of the chromosomes of an organism arranged according to their size is called
A)
karyotype
done
clear
B)
genotype
done
clear
C)
ecotype
done
clear
D)
idiogram.
done
clear
View Answer play_arrow
question_answer 172) Glucagon is not normally found in the
A)
pancreas
done
clear
B)
gastro intestinal tract
done
clear
C)
adrenal
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 173) All hypophysiotropic hormones are peptides except
A)
corticotropin releasing hormone
done
clear
B)
growth hormone
done
clear
C)
somatostatin
done
clear
D)
prolactin release inhibiting hormone.
done
clear
View Answer play_arrow
question_answer 174) Which of the following is not synthesized in both the brain arid endocrine glands?
A)
ACTH
done
clear
B)
cortisol
done
clear
C)
oxytocin
done
clear
D)
somatostatin.
done
clear
View Answer play_arrow
question_answer 175) Cumulus proligerus cells are found around
A)
oviducal funnel
done
clear
B)
corpus albicans
done
clear
C)
ovum
done
clear
D)
ovary.
done
clear
View Answer play_arrow
question_answer 176) Which of the following hormones does not have a particular target organ in the body?
A)
growth hormone
done
clear
B)
thyroxine
done
clear
C)
oxytocin
done
clear
D)
FSH.
done
clear
View Answer play_arrow
question_answer 177) Which one of the following acts as a transmitter at the neuromuscular junction?
A)
gamma aminobutyric acid
done
clear
B)
acetylcholine
done
clear
C)
thyroxine
done
clear
D)
melatonin.
done
clear
View Answer play_arrow
question_answer 178) Refractory period comes in between
A)
resting potential and depolarization
done
clear
B)
depolarization and repolarization
done
clear
C)
action potential and depolarization
done
clear
D)
two cycles of impulse conduction.
done
clear
View Answer play_arrow
question_answer 179) The helicotrema is the communicating link between the
A)
sacculus and cochlea
done
clear
B)
tympanic and vestibular canals
done
clear
C)
vestibular and median canals
done
clear
D)
sacculus and utriculus.
done
clear
View Answer play_arrow
question_answer 180) Trimethylamine oxide is an excretory product in
A)
fresh water teleosts
done
clear
B)
marine teleosts
done
clear
C)
marine invertebrate
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 181) Among HIV viruses which one is more virulent causing AIDS in 90% of the cases?
A)
HIV-I
done
clear
B)
HIV-II
done
clear
C)
HIV-III
done
clear
D)
all of these.
done
clear
View Answer play_arrow
question_answer 182) Which zone of a lake has no photosynthetic organism?
A)
profundal zone
done
clear
B)
littoral zone.
done
clear
C)
limnetic zone
done
clear
D)
both [b] and [c].
done
clear
View Answer play_arrow
question_answer 183) Terai forest is
A)
tropical forest
done
clear
B)
coniferous forest
done
clear
C)
both [a] & [b]
done
clear
D)
temperate deciduous forest.
done
clear
View Answer play_arrow
question_answer 184) The Wildlife Protection Act was enacted by the Indian Parliament in
A)
1962
done
clear
B)
1972
done
clear
C)
1983
done
clear
D)
1989.
done
clear
View Answer play_arrow
question_answer 185) During which stages (or prophase I substages) of meiosis do you expect to find the bivalents and DNA replication respectively?
A)
pachytene and interphase (between two meiotic divisions)
done
clear
B)
pachytene and interphase Gust prior to prophase I)
done
clear
C)
pachytene and S phase (of interphase just prior to prophase I)
done
clear
D)
zygotene and S phase (of interphase prior to prophase I).
done
clear
View Answer play_arrow
question_answer 186) From a diploid condition of 2N, which condition can arise due to non-disjunction of chromosomes?
A)
3N
done
clear
B)
5N
done
clear
C)
\[2N+1\]
done
clear
D)
N.
done
clear
View Answer play_arrow
question_answer 187) Which is not X-linked?
A)
Duchenne muscular dystrophy
done
clear
B)
protanopea
done
clear
C)
G6PD
done
clear
D)
congenital adrenal hyperplasia.
done
clear
View Answer play_arrow
question_answer 188) Human chromosome number was determined correctly by
A)
Landsteiner
done
clear
B)
Moorehead
done
clear
C)
Tijo and Levan
done
clear
D)
J.L. Hamerton.
done
clear
View Answer play_arrow
question_answer 189) Nucleic acids have multiple negative charges due to
A)
sugars
done
clear
B)
phosphoryl groups
done
clear
C)
associated protein
done
clear
D)
purine and pyrimidines.
done
clear
View Answer play_arrow
question_answer 190) At the end of prophase, nucleolus disappears because of
A)
its enzymatic dissolution into its macromolecules
done
clear
B)
its dispersion into cytoplasm
done
clear
C)
its dispersion into nucleoplasm
done
clear
D)
its poor stainability.
done
clear
View Answer play_arrow
question_answer 191) Waggle dance in honey bees tells about
A)
direction of food source
done
clear
B)
distance of food source
done
clear
C)
both [a] and [b]
done
clear
D)
none of the above.
done
clear
View Answer play_arrow
question_answer 192) Normal functioning of sex glands is regulated by
A)
tocopherol
done
clear
B)
calciferol
done
clear
C)
phylloquinone
done
clear
D)
pyridoxine.
done
clear
View Answer play_arrow
question_answer 193) Riboflavin contains the nucleotides
A)
FMN
done
clear
B)
FAD
done
clear
C)
both FMN and FAD
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 194) Which of the following has the highest pH?
A)
gastric juice
done
clear
B)
bile
done
clear
C)
pancreatic juice
done
clear
D)
secretion of the intestinal glands.
done
clear
View Answer play_arrow
question_answer 195) The activity of the enzyme threonine deaminase is inhibited by
A)
malonic acid
done
clear
B)
sulphur drugs
done
clear
C)
glucose-6-phosphate
done
clear
D)
isoleucine.
done
clear
View Answer play_arrow
question_answer 196) Which is not a correct situation?
A)
\[ATP\xrightarrow[{}]{~ATPase}\]3, 5, cyclic AMP
done
clear
B)
\[ATP~\xrightarrow[{}]{Adenylate\,cyclase}\] 3, 5 cyclic AMP
done
clear
C)
\[ATP~\to ADP+Pi\]
done
clear
D)
\[ADP~\to AMP+Pi\]
done
clear
View Answer play_arrow
question_answer 197) Sporozoites are
A)
uninucleate
done
clear
B)
binucleate
done
clear
C)
multinucleate
done
clear
D)
none-of these.
done
clear
View Answer play_arrow
question_answer 198) Interferon\[\beta \]is also termed as
A)
immune interferon
done
clear
B)
fibroblast interferon
done
clear
C)
leucocyte interferon
done
clear
D)
anti-immune interferon..
done
clear
View Answer play_arrow
question_answer 199) The causative agent of Hansens disease is
A)
Myobacterium tuberculosis
done
clear
B)
Myobacterium leprae
done
clear
C)
Cornybacterium diphtheriae
done
clear
D)
Clostridium tetani.
done
clear
View Answer play_arrow
question_answer 200) A pure cell line of human cancer cells used for cultivation of viruses are called
A)
malignant cells
done
clear
B)
myeloma
done
clear
C)
HeLa cells
done
clear
D)
none of these.
done
clear
View Answer play_arrow
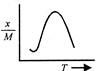
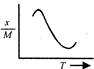
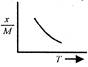
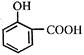
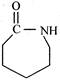
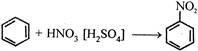 This reactive species (most likely) is
This reactive species (most likely) is