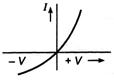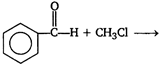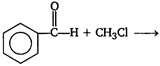question_answer 1)
A 100 turns coil kept in a magnetic field \[\overset{\to }{\mathop{\mathbf{B}}}\,=\]\[0.5\text{Wb}/{{\text{m}}^{\text{2}}},\] carries a current of 1 A. The torque acting on the coil is
A)
1.125 N-m
done
clear
B)
5.25 N-m
done
clear
C)
6.25 N-m
done
clear
D)
10 N-m
done
clear
View Answer play_arrow
question_answer 2) The ionisation potential of hydrogen atom is 13.6 V. The energy needed to ionise a hydrogen atom which is in its n = 2 state is about
A)
3.4 eV
done
clear
B)
1.5 eV
done
clear
C)
10.2 eV
done
clear
D)
13.6 eV
done
clear
View Answer play_arrow
question_answer 3) Consider the following processes (i) Electrical oscillation (ii) Rapid deceleration of fast electrons (iii)Atomic transition Which of these are likely to produce visible light ?
A)
(iii) only
done
clear
B)
(ii) and (iii)
done
clear
C)
(i) and (iii)
done
clear
D)
(i), (ii) and (iii)
done
clear
View Answer play_arrow
question_answer 4) In a series L-C circuit L = 0.405 H and\[\text{C}=\text{25}\,\mu \text{F}.\]The resistance R is zero. The frequency of resonance is
A)
30 Hz
done
clear
B)
25 Hz
done
clear
C)
50 Hz
done
clear
D)
20 Hz
done
clear
View Answer play_arrow
question_answer 5) The impedance (Z) of a circuit of frequency 50 Hz containing \[\text{R}=\text{1}0\,\Omega ,\text{C}=\text{5}0\text{ }\mu \text{F}\]in series is
A)
64.5\[\Omega \]
done
clear
B)
54.5\[\Omega \]
done
clear
C)
zero
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 6) Two electron are separated by a distance of 1 Å. The Coulombs force between them will be
A)
\[\text{2}.\text{3}\times \text{1}{{0}^{-\text{8}}}\text{ N}\]
done
clear
B)
\[\text{4}.\text{6}\times \text{1}{{0}^{-\text{8}}}\text{ N}\]
done
clear
C)
\[\text{1}.\text{15}\times \text{1}{{0}^{-8}}\text{ N}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 7) A thin oil layer floats on water. A ray of light making an angle of incidence \[40{}^\circ \] shines on oil layer. The angle of refraction of light ray in water is \[(Given,{{\mu }_{oil}}=1.45,{{\mu }_{Water}}=1.33)\]
A)
\[28.9{}^\circ \]
done
clear
B)
\[26.8{}^\circ \]
done
clear
C)
\[44.5{}^\circ \]
done
clear
D)
\[36.1{}^\circ \]
done
clear
View Answer play_arrow
question_answer 8) The refractive indices of the material of a lens for violet and red colours are respectively 1.56 and 1.54. If the focal length of the lens for violet light is 40 cm, then focal length for red colour is
A)
41.48 cm
done
clear
B)
20.25 cm
done
clear
C)
15.3 cm
done
clear
D)
2.8 cm
done
clear
View Answer play_arrow
question_answer 9) A drop of water is placed on a glass plate. A double convex lens having radius of curvature of each surface 20 cm is placed on it. The focal length of water lens \[\left( \mu =\frac{4}{3} \right),\] will be
A)
0.20 m
done
clear
B)
-0.60 m
done
clear
C)
0.30 m
done
clear
D)
0.40 m
done
clear
View Answer play_arrow
question_answer 10) A person who can see things most clearly at a distance of 10 cm requires spectacles to enable him to see clearly things at a distance of 30 cm. The focal length of the spectacles is
A)
10 cm
done
clear
B)
18 cm
done
clear
C)
30 cm
done
clear
D)
15 cm
done
clear
View Answer play_arrow
question_answer 11) A force of \[\text{1}{{\text{0}}^{\text{6}}}\text{ N}/{{\text{m}}^{\text{2}}}\]is required for breaking a material. If the density of the material is \[\text{3}\times \text{1}{{0}^{\text{3}}}\text{ kg}/{{\text{m}}^{\text{3}}},\] then what should be the length of the wire made of material, so that it breaks by its own weight?
A)
17 m
done
clear
B)
34 m
done
clear
C)
26 m
done
clear
D)
23 m
done
clear
View Answer play_arrow
question_answer 12) The surface tension of water is \[\text{7}\times \text{1}{{0}^{-\text{2}}}\text{ N}/\text{m}.\] The weight of water supported by surface tension in a capillary tube of 0.1 mm radius, will be
A)
\[\text{44}\times \text{1}{{0}^{-\text{6}}}\text{ N}\]
done
clear
B)
\[\text{22}\times \text{1}{{0}^{-\text{6}}}\text{ N}\]
done
clear
C)
\[\text{33}\times \text{1}{{0}^{-\text{6}}}\text{ N}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 13) A rain drop of radius 0.3 mm has a terminal velocity of 1 m/s in air. The viscosity of air is \[\text{18}\times \text{1}{{0}^{-5}}\] poise. The viscous force on the drop is
A)
\[16.95\times 10\]
done
clear
B)
\[1.695\times {{10}^{-2}}\]
done
clear
C)
\[\text{1}.0\text{17}\times \text{1}{{0}^{-\text{11}}}\text{ N}\]
done
clear
D)
\[\text{1}0\text{1}.\text{73}\times \text{1}{{0}^{-\text{9}}}\text{ N}\]
done
clear
View Answer play_arrow
question_answer 14) When temperature is increased from \[0{}^\circ C\] to \[273{}^\circ C\], in what ratio the average kinetic energy of molecules change ?
A)
1
done
clear
B)
3
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 15) The root mean square speed of hydrogen molecules of an ideal hydrogen gas kept in a gas chamber at 0°C is 3180 m/s. The pressure of hydrogen gas will be (density of hydrogen gas \[=\text{8}.\text{99}\times \text{1}{{0}^{-\text{2}}}\text{ kg}/{{\text{m}}^{\text{3}}}\]and 1 atm \[=\text{ 1}.0\text{1}\times \text{1}{{\text{0}}^{\text{5}}}\text{ N}/{{\text{m}}^{\text{2}}})\]
A)
3.0 atm
done
clear
B)
2.0 atm
done
clear
C)
1.0 atm
done
clear
D)
1.5 atm
done
clear
View Answer play_arrow
question_answer 16) In a thermodynamic process pressure of a fixed mass of a gas is changed in such a manner that the gas releases 20 J of heat and 8 J of work is done on the gas, if the initial internal energy of the gas was 30 J. The final internal energy will be
A)
36 J
done
clear
B)
15 J
done
clear
C)
18 J
done
clear
D)
9 J
done
clear
View Answer play_arrow
question_answer 17) A Camot engine working between 300 K and 600 K has work output of 800 J/cycle. The amount of heat energy supplied to the engine from source per cycle will be
A)
800
done
clear
B)
1600
done
clear
C)
1200
done
clear
D)
900
done
clear
View Answer play_arrow
question_answer 18) If 1 mole of a monoatomic gas \[y=\frac{5}{3}\]is mixed with 1 mole of diatomic gas \[y=\frac{7}{5}.\]The value of \[y\] for the mixture will be
A)
3
done
clear
B)
1.5
done
clear
C)
1.8
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 19) Calculate the energy radiated per minute the filament of an incandescent lamp at 2000 K. If the surface area is \[\text{5}\times \text{1}{{0}^{-5}}\text{ }{{\text{m}}^{\text{2}}}\]and its relative emittance is \[0.\text{85},\sigma =\text{ 5}.\text{7}\times {{10}^{-8}}\] MKS units
A)
1230 J
done
clear
B)
2225 J
done
clear
C)
2115 J
done
clear
D)
2315 J
done
clear
View Answer play_arrow
question_answer 20) An object is cooled from \[75{}^\circ C\] to \[65{}^\circ C\] in 2 min in a room at \[30{}^\circ C\] the time taken to cool the same object from \[55{}^\circ C\] to \[45{}^\circ C\] in the same room (in min) is
A)
7
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
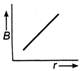
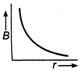
question_answer 21) The magnetic flux density B at a distance - from a long straight wire carrying a steac current varies with r as shown in figure
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 22) A satellite of mass m goes round the earth along a circular path of radius r, let Mg is the mass of the earth and Rg its radius. Then the linear speed of the satellite depends
A)
\[{{M}_{e}}\]
done
clear
B)
\[{{M}_{e}}\] only
done
clear
C)
\[\mu ,{{\mu }_{e}},V\]
done
clear
D)
\[{{M}_{e}},{{R}_{e}}\]and \[r\]
done
clear
View Answer play_arrow
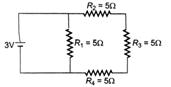
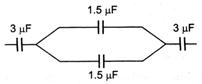
question_answer 23)
The equivalent capacitance in the given circuit is
A)
\[\text{3}\mu \text{F}\]
done
clear
B)
\[\text{1}.\text{5 }\mu \text{f}\]
done
clear
C)
\[\text{2}\mu \text{F}\]
done
clear
D)
\[1\mu \text{F}\]
done
clear
View Answer play_arrow
question_answer 24) Iron is ferromagnetic
A)
below \[770{}^\circ C\]
done
clear
B)
above \[770{}^\circ C\]
done
clear
C)
at NTP only
done
clear
D)
at all temperature
done
clear
View Answer play_arrow
question_answer 25) The component waves producing a stationary wave have amplitude, frequency and velocity are respectively 8 cm, 30 Hz and 180 cm/s. The wavelength of the wave is
A)
3 cm
done
clear
B)
6 cm
done
clear
C)
8 cm
done
clear
D)
5 cm
done
clear
View Answer play_arrow
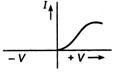
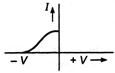
question_answer 26) Which one of the following is the graph between frequency v of the incident radiation and stopping potential?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 27) Which transition emits light of lower frequency ?
A)
\[{{\text{n}}_{\text{1}}}=\text{ 6 to }{{\text{n}}_{2}}=\text{2}\]
done
clear
B)
\[{{\text{n}}_{1}}=\text{6 to }{{\text{n}}_{2}}=1\]
done
clear
C)
\[{{\text{n}}_{\text{1}}}=\text{2 to }{{\text{n}}_{2}}=\text{6}\]
done
clear
D)
\[{{\text{n}}_{\text{1}}}=1\text{ to }{{\text{n}}_{2}}=2\]
done
clear
View Answer play_arrow
question_answer 28) Which one of the following is the weakest force ?
A)
Electrostatic force
done
clear
B)
Nuclear force
done
clear
C)
Electromagnetic force
done
clear
D)
Gravitational force
done
clear
View Answer play_arrow
question_answer 29) A ball of mass m travelling with a certain volecity has a kinetic energy KE. Then the de-Broglie wavelength associated with the ball is
A)
it is only associated with atomic particles
done
clear
B)
\[\frac{h}{2KEm}\]
done
clear
C)
\[\sqrt{\frac{h}{2KEm}}\]
done
clear
D)
\[\frac{h}{\sqrt{2KEm}}\]
done
clear
View Answer play_arrow
question_answer 30) Work function of three metals A, B, C are 4.5 eV, 4.3 eV, 3.5 eV respectively. If a light of wavelength \[4000\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\] is incident on the metals, then
A)
photoelectrons emitted from C
done
clear
B)
photoelectrons are emitted from A
done
clear
C)
photoelectrons emitted from B
done
clear
D)
photoelectrons emitted from all the surfaces
done
clear
View Answer play_arrow
question_answer 31) The time period of a body suspended by a spring is T. The new time period when the spring is cut into two equal parts and body is suspended by one part is
A)
\[\sqrt{2}T\]
done
clear
B)
\[\frac{T}{\sqrt{2}}\]
done
clear
C)
\[\frac{T}{\sqrt{3}}\]
done
clear
D)
\[\sqrt{3}T\]
done
clear
View Answer play_arrow
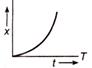
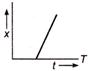
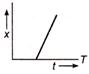
question_answer 32) Which of the following distance-time graph represents one dimensions at uniform motion?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 33)
Which logic gate is represented by following diagram ?
A)
XOR
done
clear
B)
NOR
done
clear
C)
OR
done
clear
D)
AND
done
clear
View Answer play_arrow
question_answer 34) A 220 V, 50 Hz AC source is connected to an inductance of 0.2 H and resistance of 20 tl in series. The current in the circuit is
A)
3.33 A
done
clear
B)
33.3 A
done
clear
C)
10 A
done
clear
D)
5 A
done
clear
View Answer play_arrow
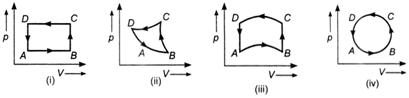
question_answer 35)
In the diagrams (i) to (iv), variation of volume by changing pressure is shown. A gas is taken along the pathABCDA.
A)
positive in all the cases (i) to (iv)
done
clear
B)
positive in cases (i), (ii) and (iii) but zero in case (iv)
done
clear
C)
negative in cases (i), (ii) and (iii) but zero in case (iv)
done
clear
D)
zero in all the four cases
done
clear
View Answer play_arrow
question_answer 36)
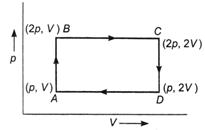
An ideal monoatomic gas is taken round the cycle ABCDA as shown in the figure. The work done during the cycle is
A)
zero
done
clear
B)
\[\frac{pV}{2}\]
done
clear
C)
\[2pV\]
done
clear
D)
\[pV\]
done
clear
View Answer play_arrow
question_answer 37) Which one of the following does not support the wave nature of light ?
A)
Photoelectric effect
done
clear
B)
Polarisation
done
clear
C)
Diffraction
done
clear
D)
Interference
done
clear
View Answer play_arrow
question_answer 38)
The value of current I in the circuit shown in the
A)
0.2 A
done
clear
B)
0.8 A
done
clear
C)
1.5 A
done
clear
D)
2.4 A
done
clear
View Answer play_arrow
question_answer 39) A toy car travels in a horizontal circle of radius 2a, kept on the track by a radial elastic string of unstretched length a. The period of rotation is T. Now the car is speeded up until it is moving in a circle of radius 3a. Assuming that the string obeys Hookes law then the new period will be
A)
\[\sqrt{\frac{4}{3}}T\]
done
clear
B)
\[\frac{{{3}^{2}}}{{{4}^{2}}}T\]
done
clear
C)
\[\frac{\sqrt{3}}{2}T\]
done
clear
D)
\[\frac{3}{4}T\]
done
clear
View Answer play_arrow
question_answer 40) We have a galvanometer of resistance 25 n. It is shunted by a 2.5 n wire, the part of total current that flows through the galvanometer is given as
A)
\[\frac{{{I}_{g}}}{I}=\frac{4}{11}\]
done
clear
B)
\[\frac{{{I}_{g}}}{I}=\frac{3}{11}\]
done
clear
C)
\[\frac{{{I}_{g}}}{I}=\frac{2}{11}\]
done
clear
D)
\[\frac{{{I}_{g}}}{I}=\frac{1}{11}\]
done
clear
View Answer play_arrow
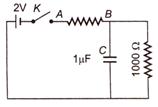
question_answer 41)
When the key is pressed at time t = 0 then which of the following statement about the current I on the resistor AB of the given circuit is true ?
A)
at t = 0, \[I\]= 2 mA and with time it goes to 1mA
done
clear
B)
J oscillates between 1 mA and 2 mA
done
clear
C)
J = 2 mA at all t
done
clear
D)
I = 1 mA at all t
done
clear
View Answer play_arrow
question_answer 42) Eight drops of equal size are falling through aL- with steady velocity of 10 cm/s. If the drop; coalesce, what could be its terminal velocity
A)
40 cm/s
done
clear
B)
20 cm/s
done
clear
C)
25 cm/s
done
clear
D)
50 cm/s
done
clear
View Answer play_arrow
question_answer 43) Light of two different frequency whose photon have energies 1 eV and 2.5 eV respectively successively illuminate a metal of work function 0.5 eV. The ratio of maximum speed of the emitted electron will be
A)
1 : 1
done
clear
B)
1 : 2
done
clear
C)
1 : 4
done
clear
D)
1 : 5
done
clear
View Answer play_arrow
question_answer 44) If the pitch of the sound of a source appears to drop by 10% to a moving person, then the velocity of motion is
A)
20 m/s
done
clear
B)
33 m/s
done
clear
C)
40 m/s
done
clear
D)
50 m/s
done
clear
View Answer play_arrow
question_answer 45) By opening the door of a refrigerator which is inside a room, the temperature of room
A)
first decreases then increases
done
clear
B)
remains unchanged
done
clear
C)
increases
done
clear
D)
decreases
done
clear
View Answer play_arrow
question_answer 46) A vessel has 6 g of oxygen of pressure p and temperature 400 K, a small hole is made in it so that oxygen leaks out. How much oxygen leaks out, if the final pressure isp/2 and temperature is 300 K ?
A)
3 g
done
clear
B)
2 g
done
clear
C)
4 g
done
clear
D)
5 g
done
clear
View Answer play_arrow
question_answer 47) The maximum acceleration of the body executing SHM is Og and maximum velocity is vq the amplitude is given by
A)
\[\frac{1}{{{a}_{0}}{{v}_{0}}}\]
done
clear
B)
\[\frac{{{a}_{0}}^{2}}{{{v}_{0}}}\]
done
clear
C)
\[{{v}_{a}}{{a}_{0}}\]
done
clear
D)
\[\frac{{{v}_{a}}^{2}}{{{a}_{0}}}\]
done
clear
View Answer play_arrow
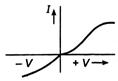
question_answer 48) Different voltage are applied across a p-n junction and the currents are measured for each value. Which of the following graphs is obtained between voltage V and current ?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
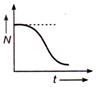
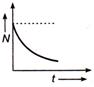
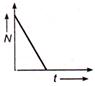
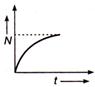
question_answer 49) Which of the following graph represents the variation of activity N of a radioactive substance with time t ?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
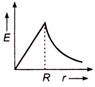
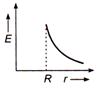
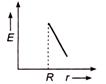
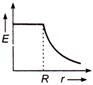
question_answer 50) Which one of the graphs represents the variation of electric field strength \[E\] with distance \[r\]from the centre of a uniformly charged conducting sphere ?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
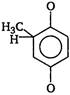
question_answer 51) Acetophenone \[(Ph-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}})\] is prepared in the laboratory by
A)
done
clear
B)
done
clear
C)
done
clear
D)
\[Ph-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-Cl+C{{H}_{4}}\xrightarrow{AlC{{l}_{3}}}\]
done
clear
View Answer play_arrow
question_answer 52) The energy of hydrogen n atom in its ground state is -13.6 eV. The energy of the level corresponding to the quantum number n = 5 is
A)
-5.40 eV
done
clear
B)
-0.54 eV
done
clear
C)
-2.72 eV
done
clear
D)
-0.85 eV
done
clear
View Answer play_arrow
question_answer 53) Which of the following species is diamagnetic?
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{O}_{2}}^{2-}\]
done
clear
C)
\[O_{2}^{-}\]
done
clear
D)
\[{{O}_{2}}^{+}\]
done
clear
View Answer play_arrow
question_answer 54) The rate of diffusion of a gas is proportional to
A)
\[\frac{p}{d}\]
done
clear
B)
\[\sqrt{\frac{p}{d}}\]
done
clear
C)
\[\frac{pV}{M}\]
done
clear
D)
\[\sqrt{\frac{pV}{d}}\]
done
clear
View Answer play_arrow
question_answer 55) Which of the following hydrides is electron deficient molecule?
A)
NaH
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[Ca{{H}_{2}}\]
done
clear
D)
\[{{B}_{2}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 56) Oxidation number of Cr in \[Cr{{O}_{6}}\] is
A)
+8
done
clear
B)
+6
done
clear
C)
+4
done
clear
D)
+10
done
clear
View Answer play_arrow
question_answer 57) Which of the following solutions boils at the highest temperature?
A)
0.1 M NaCI
done
clear
B)
0.1 M urea
done
clear
C)
0.1 M \[BaC{{l}_{2}}\]
done
clear
D)
0.1 M glucose
done
clear
View Answer play_arrow
question_answer 58) For treatment of cancerous tumours, the radio isotope used was
A)
U - 235
done
clear
B)
Co - 60
done
clear
C)
P - 32
done
clear
D)
Pu - 239
done
clear
View Answer play_arrow
question_answer 59) The main structural feature of protein is
A)
the ester linkage
done
clear
B)
the ether linkage
done
clear
C)
the peptide linkage
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 60) Which one of the following acids exhibits optical activity?
A)
Lactic acid
done
clear
B)
Benzoic acid
done
clear
C)
Oxalic acid
done
clear
D)
Acetic acid
done
clear
View Answer play_arrow
question_answer 61) What is the product B in the following reaction sequence\[C{{H}_{3}}-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow{KCN/{{H}_{2}}S{{O}_{4}}}A\xrightarrow{LiAl{{H}_{4}}}B?\]
A)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,H-COOH\]
done
clear
B)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,}}\,-COOH\]
done
clear
C)
\[C{{H}_{3}}-\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,}}\,-C{{H}_{2}}NH\]
done
clear
D)
\[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,N-CN\]
done
clear
View Answer play_arrow
question_answer 62) Which one of the following acids will undergo easy thermal decarboxylation?
A)
\[C{{H}_{3}}.\,C{{H}_{2}}COOH\]
done
clear
B)
\[HOOCC{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{2}}COOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}COOH\]
done
clear
View Answer play_arrow
question_answer 63) The gas which combine with haemoglobin to damage its oxygen carrying capacity is
A)
\[C{{O}_{2}}(g)\]
done
clear
B)
\[{{O}_{2}}(g)\]
done
clear
C)
\[CO\,(g)\]
done
clear
D)
\[{{N}_{2}}(g)\]
done
clear
View Answer play_arrow
question_answer 64) Which one of the following is obtained when acetone is treated with bleaching powder?
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
C)
\[CHC{{l}_{3}}\]
done
clear
D)
\[CC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 65) Benzyl alcohol is obtained from Benzaldehyde by
A)
Fittig reaction
done
clear
B)
reduction with \[LiAl{{H}_{4}}\]
done
clear
C)
Cannizaros reaction
done
clear
D)
Both (a) and (b)
done
clear
View Answer play_arrow
question_answer 66) An isocyanide on hydrolysis gives
A)
n-substituted amide
done
clear
B)
\[{{1}^{o}}\]-amine and formic acid
done
clear
C)
carboxylic acid and ammonia
done
clear
D)
an amide
done
clear
View Answer play_arrow
question_answer 67) \[C{{H}_{3}}CH=CHCHO\] is oxidised to \[C{{H}_{3}}CH=CHCOOH\] using
A)
osmium tetraoxide
done
clear
B)
selenium dioxide
done
clear
C)
ammoniacal silver nitrate
done
clear
D)
alkaline permanganate
done
clear
View Answer play_arrow
question_answer 68) The reagent with which both acetaldehyde and acetone react easily is
A)
Schiff s reagent
done
clear
B)
Tollens reagent
done
clear
C)
Grignard reagent
done
clear
D)
Fehling solution
done
clear
View Answer play_arrow
question_answer 69) The reaction sequence\[C{{H}_{3}}C{{H}_{2}}COOH\xrightarrow{SOC{{l}_{2}}}A\]\[\xrightarrow{Pd/BaS{{O}_{4}}}B\xrightarrow{HOC{{H}_{2}}C{{H}_{2}}OH}C\]C is most likely to be
A)
done
clear
B)
done
clear
C)
\[C{{H}_{3}}-C{{H}_{2}}-CHO\]
done
clear
D)
done
clear
View Answer play_arrow
question_answer 70) When phenol is heated with KOH and chloroform, it forms
A)
salicylaldehyde
done
clear
B)
benzoic acid
done
clear
C)
potassium phenolate
done
clear
D)
salicyclic acid
done
clear
View Answer play_arrow
question_answer 71) 3-methyl-2-butanol on treatment with \[HCl\]gives predominantly
A)
2-chloro-3-methyl butane
done
clear
B)
2, 2-dimethyl pentane
done
clear
C)
2-chloro-2-methyl butane
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 72) Percentage composition of an organic compound is as follows\[C=10.06,\,\,\,\,H=0.84,\,\,\,Cl=89.10\] Which of the following correspond to its molecular formula if the vapour density is 60.0?
A)
\[C{{H}_{3}}Cl\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[C{{H}_{2}}C{{l}_{2}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 73) In the second group of qualitative analysis, \[{{H}_{2}}S\] is passed through a solution acidified with \[HCl\] in order to
A)
increase the concentration of \[{{S}^{2-}}\]ions
done
clear
B)
limit the concentration of \[{{S}^{2-}}\]ions
done
clear
C)
add the \[C{{l}^{-}}\] ions
done
clear
D)
increase the solubility of \[{{H}_{2}}S\]
done
clear
View Answer play_arrow
question_answer 74) Synthetic detergents are more effective in hard water than soaps because
A)
they are non-ionic
done
clear
B)
their \[C{{a}^{2+}}\] and \[M{{g}^{2+}}\] salts are insoluble in water
done
clear
C)
their \[C{{a}^{2+}}\] and \[M{{g}^{2+}}\] salts are water soluble
done
clear
D)
they are highly soluble in water
done
clear
View Answer play_arrow
question_answer 75) \[\underset{sucrose}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,(s)+12{{O}_{2}}\xrightarrow{{}}12C{{O}_{2}}(g)\]\[+11{{H}_{2}}O(l)\] \[\Delta H=-5.65\times {{10}^{3}}\,kJ\] Complete combustion of 1.00 kg sucrose is done, heat evolved will be
A)
\[+3.51\times {{10}^{5}}kJ\]
done
clear
B)
\[+7.38\times {{10}^{5}}kJ\]
done
clear
C)
\[+5.25\times {{10}^{3}}kJ\]
done
clear
D)
\[+1.65\times {{10}^{4}}kJ\]
done
clear
View Answer play_arrow
question_answer 76) Atoms or ion missing from normal lattice point creating a vacancy due to
A)
Frenkel defect
done
clear
B)
mass defect
done
clear
C)
Schottky defect
done
clear
D)
interstitial defect
done
clear
View Answer play_arrow
question_answer 77) At constant T and p which one of the following statements is correct for the reaction? \[CO(g)+\frac{1}{2}{{O}_{2}}(g)\xrightarrow{{}}C{{O}_{2}}(g)\]
A)
\[\Delta H>\Delta E\]
done
clear
B)
\[\Delta H<\Delta E\]
done
clear
C)
\[\Delta H=\Delta E\]
done
clear
D)
\[\Delta H\] is independent of physical state of reactants
done
clear
View Answer play_arrow
question_answer 78) A gas occupies 2 L at STP. It is provided 300 J heat so that its volume becomes 2.5 L at 1 arm. Calculate the change in internal energy
A)
352.49 J
done
clear
B)
849.35 J
done
clear
C)
249.35 J
done
clear
D)
302.98 J
done
clear
View Answer play_arrow
question_answer 79) The isotope \[y{{A}^{x}}\] undergoes a series of ma and \[n\beta \] disintegrations to form a stable isotope \[{{y}_{-10}}{{B}^{x-32}}\]. The value of m and n are
A)
5 and 8
done
clear
B)
6 and 8
done
clear
C)
8 and 6
done
clear
D)
8 and 10
done
clear
View Answer play_arrow
question_answer 80) The activity of a radioactive element falls to \[\left( \frac{1}{32} \right)\] of its original value in 50 days. The \[{{t}_{1/2}}\] of the substance is
A)
20 days
done
clear
B)
15 days
done
clear
C)
10 days
done
clear
D)
5 days
done
clear
View Answer play_arrow
question_answer 81) If the equilibrium concentration of A is doubled in the following equilibrium \[A{{B}_{2}}(s)A(g)+{{B}_{2}}(g)\]the equilibrium concentration of \[{{B}_{2}}\] would be
A)
one fourth of initial concentration
done
clear
B)
half of the initial concentration
done
clear
C)
doubled
done
clear
D)
same as initial concentration
done
clear
View Answer play_arrow
question_answer 82) The half-life of a radioactive isotope is 3 h. If the initial mass of the isotope was 300 g, the mass which remains undecayed in 18 h would be
A)
2.34 g
done
clear
B)
1.17 g
done
clear
C)
9.36 g
done
clear
D)
4.68 g
done
clear
View Answer play_arrow
question_answer 83) At \[{{298}^{o}}K\] standard reduction potential of half reduction reactions are given below, which of the following is the best suited to be used as anode?
A)
\[2{{H}^{1+}}=2{{e}^{-}}\xrightarrow{{}}{{H}_{2}}(g)\,(+0.000)\]
done
clear
B)
\[Z{{n}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Zn(s)\,(-0.762)\]
done
clear
C)
\[F{{e}^{3+}}+{{e}^{-}}\xrightarrow{{}}F{{e}^{2+}}(s)\,\,(-0.44)\]
done
clear
D)
\[C{{r}^{2+}}+2{{e}^{-}}\xrightarrow{{}}Cr(s)\,\,(-0.540)\]
done
clear
View Answer play_arrow
question_answer 84) Which of the following reactions occurs at the cathode?
A)
\[S{{n}^{2+}}\xrightarrow{{}}S{{n}^{4+}}+2{{e}^{-}}\]
done
clear
B)
\[A{{g}^{+}}+\,\,{{e}^{-}}\xrightarrow{{}}Ag\]
done
clear
C)
\[Zn\xrightarrow{{}}Z{{n}^{2+}}+2{{e}^{-}}\]
done
clear
D)
\[2O{{H}^{-}}\xrightarrow{{}}{{H}_{2}}O+\frac{1}{2}{{O}_{2}}+2{{e}^{-}}\]
done
clear
View Answer play_arrow
question_answer 85) To deposit 0.634 g of copper by electrolysis of aqueous cupric sulphate solution, the amount of electricity required (in coulombs) is
A)
1930
done
clear
B)
3960
done
clear
C)
4825
done
clear
D)
9650
done
clear
View Answer play_arrow
question_answer 86) Some of the polar crystal when heated produce electric current. This phenomena is termed as
A)
piezoelectric effect
done
clear
B)
anti-ferroelectric effect
done
clear
C)
ferroelectric effect
done
clear
D)
Pyro electric effect
done
clear
View Answer play_arrow
question_answer 87) Pick the correct name of \[[Co{{(N{{H}_{3}})}_{5}}Cl]C{{l}_{2}}\]
A)
chloropentammine cobalt (II) chloride
done
clear
B)
pentammine chloro cobalt (III) chloride
done
clear
C)
chloropentammine cobalt (III)
done
clear
D)
pentammine cobalt (III) chloride
done
clear
View Answer play_arrow
question_answer 88) Which one of the following statements concerning lanthanides elements is false?
A)
Most characteristic oxidation state of lanthanides elements is +3
done
clear
B)
All lanthanides are metals
done
clear
C)
The ionic radii of trivalent lanthanide steadily increase with increase in atomic number
done
clear
D)
Lanthanides are separated from one another by ion exchange methods
done
clear
View Answer play_arrow
question_answer 89) Which has maximum paramagnetic nature?
A)
\[[Fe{{(CN)}_{6}}{{[}^{4-}}\]
done
clear
B)
\[{{[Cu{{({{H}_{2}}O)}_{4}}]}^{2+}}\]
done
clear
C)
\[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
done
clear
D)
\[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 90) The oxidation state of Ni in Ni \[{{(CO)}_{4}}\]is
A)
zero
done
clear
B)
-2
done
clear
C)
+2
done
clear
D)
+4
done
clear
View Answer play_arrow
question_answer 91) The number of moles of \[KMn{{O}_{4}}\] that will be needed to react completely with one mole of ferrous oxalate in acidic solution is
A)
4/5
done
clear
B)
3/5
done
clear
C)
2/5
done
clear
D)
1
done
clear
View Answer play_arrow
question_answer 92) Bronze is an alloy of
A)
copper, zinc and nickel
done
clear
B)
copper, zinc
done
clear
C)
copper, zinc and tin
done
clear
D)
copper and tin
done
clear
View Answer play_arrow
question_answer 93) Aluminium hydroxide is soluble in excess of sodium hydroxide forming the ion
A)
\[Al{{O}_{3}}^{-}\]
done
clear
B)
\[Al{{O}_{2}}^{3+}\]
done
clear
C)
\[Al{{O}_{2}}^{3-}\]
done
clear
D)
\[Al{{O}_{2}}^{-}\]
done
clear
View Answer play_arrow
question_answer 94) Identify the reaction not feasible among the following
A)
\[2NaOH\xrightarrow{\Delta }N{{a}_{2}}O+{{H}_{2}}O\]
done
clear
B)
\[2LiOH\xrightarrow{\Delta }L{{i}_{2}}O+{{H}_{2}}O\]
done
clear
C)
\[MgC{{O}_{3}}\xrightarrow{\Delta }MgO+C{{O}_{2}}\]
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 95) In the qualitative analysis of nitrate in ring test a brown ring is formed due to the formation of
A)
\[{{N}_{2}}O\,.\,FeS{{O}_{4}}\]
done
clear
B)
\[FeS{{O}_{4}}NO\]
done
clear
C)
\[N{{O}_{2}}\]
done
clear
D)
\[FeS{{O}_{4}}N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 96) An element having electronic configuration \[1{{s}^{2}},2{{s}^{2}},2{{p}^{6}},3{{s}^{2}}3{{p}^{1}}\] will form
A)
neutral oxide
done
clear
B)
acidic oxide
done
clear
C)
basic oxide
done
clear
D)
amphoteric oxide
done
clear
View Answer play_arrow
question_answer 97) Allylic bromination is carried out by
A)
n-bromosuccinimide
done
clear
B)
\[B{{r}_{2}}/C{{S}_{2}}\]
done
clear
C)
\[HBr/{{H}_{2}}{{O}_{2}}\]
done
clear
D)
\[HOBr\]
done
clear
View Answer play_arrow
question_answer 98) MO configuration of He is
A)
\[{{(\sigma 1s)}^{2}},{{(\overset{*}{\mathop{\sigma }}\,1s)}^{2}},{{(\sigma 2s)}^{1}}\]
done
clear
B)
\[{{(\sigma 1s)}^{2}},{{(\overset{*}{\mathop{\sigma }}\,1s)}^{2}},{{(\overset{*}{\mathop{\sigma }}\,2s)}^{1}}\]
done
clear
C)
\[{{(\sigma 1s)}^{2}},{{(\overset{*}{\mathop{\sigma }}\,1s)}^{2}},{{(\sigma 2s)}^{2}}\]
done
clear
D)
\[{{(\sigma 1s)}^{2}},{{(\overset{*}{\mathop{\sigma }}\,1s)}^{2}},{{(\sigma 2s)}^{1}}\]
done
clear
View Answer play_arrow
question_answer 99) Most stable carbonium ion is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 100) Which of the following has maximum number or unpaired electrons?
A)
\[C{{u}^{+}}\]
done
clear
B)
Zn
done
clear
C)
\[F{{e}^{2+}}\]
done
clear
D)
\[N{{i}^{3+}}\]
done
clear
View Answer play_arrow
question_answer 101) A group of compounds now recognised as local hormones are
A)
kinins
done
clear
B)
prostacyclins
done
clear
C)
cytokines
done
clear
D)
substance- P
done
clear
View Answer play_arrow
question_answer 102) Which one of the following is an example of glycoprotein?
A)
Haemoglobin
done
clear
B)
Lecithin
done
clear
C)
Mucin
done
clear
D)
Casein
done
clear
View Answer play_arrow
question_answer 103) Chitin is a structural polysaccharide and is polymerised from
A)
glucose
done
clear
B)
ribose
done
clear
C)
deoxyribose
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 104) The most important essential fatty acid is
A)
linoleic acid
done
clear
B)
linolenic acid
done
clear
C)
arachidonic acid
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 105) During chemical evolution key biological compounds were synthesized
A)
in the atmosphere
done
clear
B)
along the ocean shore
done
clear
C)
in the ocean
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 106) Which of the following statements is correct?
A)
\[N{{a}^{+}}\]ions help in the conduction of nerve impulses
done
clear
B)
\[N{{a}^{+}}\]ions are important in transport of substances across membranes
done
clear
C)
\[N{{a}^{+}}\]ions help to retain water in the body
done
clear
D)
\[N{{a}^{+}}\]ions are associated with all the above functions
done
clear
View Answer play_arrow
question_answer 107) Which of the following statements is incorrect?
A)
The living and non-living are made up of the same elements
done
clear
B)
Life originated in air
done
clear
C)
Energy transfers and transformations do not occur continuously in a living cell
done
clear
D)
ATP is considered as the energy currency of a cell
done
clear
View Answer play_arrow
question_answer 108) Which of the following statements is incorrect?
A)
The spermatids differentiate into structurally complex spermatozoa by the process of spermiogenesis
done
clear
B)
Fertilization is a physico-chemical event
done
clear
C)
Cleavage transforms the fertilized ovum into a sphere of aggregated blastomeres
done
clear
D)
Cleavage divisions bring appreciable increase in the mass of protoplasm in the developing embryo
done
clear
View Answer play_arrow
question_answer 109) In the event of pregnancy, the corpus luteum persists under the influence of
A)
LH
done
clear
B)
FSH
done
clear
C)
chorionic gonadotropin
done
clear
D)
progesterone
done
clear
View Answer play_arrow
question_answer 110) Reabsorption of \[N{{a}^{+}}\]is controlled by which One of the following hormones?
A)
Aldosterone
done
clear
B)
Oestrogen
done
clear
C)
Glucocorticoids
done
clear
D)
Testosterone
done
clear
View Answer play_arrow
question_answer 111) In the tissues, high concentration of carbondioxide
A)
increases the affinity of haemoglobin to both oxygen and hydrogen
done
clear
B)
increases the affinity of haemoglobin to oxygen but decreases its affinity to hydrogen
done
clear
C)
decreases the affinity of haemoglobin to oxygen but increases its affinity to hydrogen
done
clear
D)
decreases the affinity of haemoglobin to both oxygen and hydrogen
done
clear
View Answer play_arrow
question_answer 112) The colon can actively absorb
A)
sodium
done
clear
B)
glucose
done
clear
C)
amino acids
done
clear
D)
glycerol
done
clear
View Answer play_arrow
question_answer 113) The colloid osmotic pressure of blood is due to
A)
albumin
done
clear
B)
globulin
done
clear
C)
fibrinogen
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 114) In a normal ECG recording heart sound will occur
A)
on the P wave and at the end of QRS complex
done
clear
B)
on the QRS complex and at the end of T wave
done
clear
C)
at the end of P wave and on the T wave
done
clear
D)
at the end of QRS complex and on T wave
done
clear
View Answer play_arrow
question_answer 115) Release of a neurotransmitter at a synapric junction is brought about by
A)
sodium
done
clear
B)
potassium
done
clear
C)
chloride
done
clear
D)
calcium
done
clear
View Answer play_arrow
question_answer 116) Accumulation of uric acid crystals in the synovial joints causes
A)
rheumatoid arthritis
done
clear
B)
osteo - arthritis
done
clear
C)
gout
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 117) The so called tertian periodicity of Plasmodium vivox refers to the
A)
pre- erythrocytic phase
done
clear
B)
post- erythrocytic phase
done
clear
C)
erythrocytic phase
done
clear
D)
sexual phase
done
clear
View Answer play_arrow
question_answer 118) Amoebiasis in human is acquired through
A)
viral infection
done
clear
B)
contamination of food with cysts
done
clear
C)
accidental consumption of amoebic trophozoites
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 119) Kala- azar is transmitted by
A)
mosquito
done
clear
B)
sandfly
done
clear
C)
flea
done
clear
D)
lice
done
clear
View Answer play_arrow
question_answer 120) Which of the following is not an autosomal recessive trait?
A)
Phenylketonuria
done
clear
B)
Cystic fibrosis
done
clear
C)
Albinism
done
clear
D)
Polydactyly
done
clear
View Answer play_arrow
question_answer 121) The common means of transmission of AIDS is
A)
sexual intercourse
done
clear
B)
blood transfusion
done
clear
C)
placental transfer
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 122) A gene that has multiple effects is called
A)
dominant gene
done
clear
B)
mutant gene
done
clear
C)
pleiotropic gene
done
clear
D)
operator gene
done
clear
View Answer play_arrow
question_answer 123) The vector of ?break bone fever? is
A)
Culex sp.
done
clear
B)
Aedes sp.
done
clear
C)
Anopheles sp.
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 124) Which of the following is the major immunoglobulin of human serum?
A)
IgG
done
clear
B)
IgM
done
clear
C)
IgA
done
clear
D)
IgE
done
clear
View Answer play_arrow
question_answer 125) Epidemic dropsy is caused by the contamination of mustard oil with
A)
Argemone
done
clear
B)
Aspergillus
done
clear
C)
Crotalaria
done
clear
D)
Fusarium
done
clear
View Answer play_arrow
question_answer 126) The population dynamics of a species is basically regulated by
A)
climate
done
clear
B)
food supply
done
clear
C)
competition between and within the species
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 127) "Algal zone" is found in
A)
normal roots of Cycas
done
clear
B)
normal roots of Pinus
done
clear
C)
coralloid roots of Cycas
done
clear
D)
stem of Cycas
done
clear
View Answer play_arrow
question_answer 128) Rickettsiae is a group of
A)
viruses
done
clear
B)
bacteria
done
clear
C)
fungi
done
clear
D)
PPLO
done
clear
View Answer play_arrow
question_answer 129) The bacteria oxidising a number of inorganic compounds to obtain energy for the assimilation of CO2 are called
A)
photoautotrophic bacteria
done
clear
B)
chemoautotrophic bacteria
done
clear
C)
heterotrophic bacteria
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 130) Which of the following statement is correct?
A)
Middle lamella is actually the blue black colored wall
done
clear
B)
Middle lamella is the wall which keeps the two adjacent cells together
done
clear
C)
Middle lamella is a wall between cells
done
clear
D)
Middle lamella is a material which keeps adjacent cells together
done
clear
View Answer play_arrow
question_answer 131) Cycas and Ginkgo are regarded as primitive gymnosperms because
A)
they lack cones
done
clear
B)
they need water for fertilization
done
clear
C)
they are living fossils
done
clear
D)
they have ciliated sperms
done
clear
View Answer play_arrow
question_answer 132) Under stress, plants produce larger quantities of
A)
auxin
done
clear
B)
abscisic acid
done
clear
C)
cytokinin
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 133) If the stoma remains surrounded by a limited number of cells which cannot be distinguished from other epidermal cells. This type of stomata is known as
A)
perigynous type
done
clear
B)
paracytic type
done
clear
C)
anisocytic type
done
clear
D)
anomocytic type
done
clear
View Answer play_arrow
question_answer 134) A long day plant flowers only if exposed to a light period
A)
more than its critical day length
done
clear
B)
less than its critical day length
done
clear
C)
equal to its critical day length
done
clear
D)
slightly less than its critical day length
done
clear
View Answer play_arrow
question_answer 135) An important example of pleiotropy is
A)
thalassaemia
done
clear
B)
sickle cell anaemia
done
clear
C)
haemophilia
done
clear
D)
leukaemia
done
clear
View Answer play_arrow
question_answer 136) Mosaic symptom is commonly found in
A)
bacterial disease
done
clear
B)
fungal disease
done
clear
C)
viral disease
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 137) The commercial cork is obtained from
A)
jamun
done
clear
B)
pine
done
clear
C)
oak
done
clear
D)
teak
done
clear
View Answer play_arrow
question_answer 138) Expanded green stem of Opuntia is called
A)
phylloclade
done
clear
B)
tendril
done
clear
C)
bulbs
done
clear
D)
cladode
done
clear
View Answer play_arrow
question_answer 139) Diatoms float in water because they have
A)
flagella on the one end of the body
done
clear
B)
flagella on the both ends of the body
done
clear
C)
wing like structure in middle of the body
done
clear
D)
light storage lipids in the body
done
clear
View Answer play_arrow
question_answer 140) Which of the following is an insectivorous plant?
A)
Nepenthes
done
clear
B)
Solarium
done
clear
C)
Cuscuta
done
clear
D)
Asparagus
done
clear
View Answer play_arrow
question_answer 141) When the root is swollen in the middle and tapers at both ends, it will be called as
A)
tuberous root
done
clear
B)
conical root
done
clear
C)
fusiform root
done
clear
D)
napiform root
done
clear
View Answer play_arrow
question_answer 142) In tissue culture of parenchyma, mitosis are accelerated in the presence of
A)
auxin only
done
clear
B)
cytokinin only
done
clear
C)
Both (a) and (b)
done
clear
D)
gibberellin and auxin
done
clear
View Answer play_arrow
question_answer 143) In longitudinal section of root tip various regions of growth may be seen in sequence from top towards root cap as
A)
cell division, cell elongation, cell maturation
done
clear
B)
cell maturation, cell elongation, cell division
done
clear
C)
cell elongation, cell maturation, cell division
done
clear
D)
cell maturation, cell division, cell elongation
done
clear
View Answer play_arrow
question_answer 144) The largest flower found is known as
A)
Rafflesia
done
clear
B)
Tecoma
done
clear
C)
Musa
done
clear
D)
Cauliflower
done
clear
View Answer play_arrow
question_answer 145) The genera that lacks cotyledons but is placed with dictoyledonous plants, in classification is
A)
Datura
done
clear
B)
Capsicum
done
clear
C)
Triticum
done
clear
D)
Cuscuta
done
clear
View Answer play_arrow
question_answer 146) Vascular cambium never develops in the stem of
A)
banana
done
clear
B)
guava
done
clear
C)
mango
done
clear
D)
sunflower
done
clear
View Answer play_arrow
question_answer 147) If the number of chromosomes in the cells of nucellus in Pinus is 24, what would be the number of chromosomes in megaspore mother cell
A)
72
done
clear
B)
24
done
clear
C)
12
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 148) A fibrous root system is best adapted to perform which one of the following functions?
A)
Storage of food
done
clear
B)
Anchorage of the plant into the soil
done
clear
C)
Absorption of water and minerals from the soil
done
clear
D)
Transport of water and organic food
done
clear
View Answer play_arrow
question_answer 149) A physiological response to the duration of light and darkness is a
A)
daily phase cycle
done
clear
B)
circadian rhythm
done
clear
C)
biological clock
done
clear
D)
photoperiodism
done
clear
View Answer play_arrow
question_answer 150) Important estuaries of India are located in
A)
West Bengal
done
clear
B)
Tamil Nadu
done
clear
C)
Both (a) and (b)
done
clear
D)
West Bengal, Tamil Nadu and Orissa
done
clear
View Answer play_arrow
question_answer 151) The type of cells that are predominant in potato tubers
A)
phloem cells
done
clear
B)
spongy cells
done
clear
C)
parenchyma cells
done
clear
D)
parenchyma and collenchyma cells
done
clear
View Answer play_arrow
question_answer 152) Tonoplast is a membrane, which is found surrounding the
A)
vacuole
done
clear
B)
cytoplasm
done
clear
C)
nucleus
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 153) The tallest plant out of the following is
A)
Sequoia
done
clear
B)
Eucalyptus
done
clear
C)
Pinus
done
clear
D)
Casuarina
done
clear
View Answer play_arrow
question_answer 154) A pair of guard cells form a
A)
lenticel
done
clear
B)
stoma
done
clear
C)
hydathode
done
clear
D)
laticifer
done
clear
View Answer play_arrow
question_answer 155) The grittiness of the pulp guava fruit is due to presence of
A)
sclereids
done
clear
B)
tracheids
done
clear
C)
fibres
done
clear
D)
companion cells
done
clear
View Answer play_arrow
question_answer 156) "Foolish seedling disease" of rice in Japan was caused by
A)
the deficiency of nitrogen
done
clear
B)
a bacterium
done
clear
C)
a fungus
done
clear
D)
a virus
done
clear
View Answer play_arrow
question_answer 157) Which one is the causative organism of rust disease in wheat?
A)
Puccinia
done
clear
B)
Aspergillus
done
clear
C)
Rhizopus
done
clear
D)
Neurospora
done
clear
View Answer play_arrow
question_answer 158) The rod shaped cells of Bacillus megatarium are converted to spherical units called protoplast on treatment with the enzyme
A)
lysozyme
done
clear
B)
cellulose
done
clear
C)
cellulase and hemicellulase
done
clear
D)
cellulase, hemicellulase and pectinase
done
clear
View Answer play_arrow
question_answer 159) Tropical plants like sugarcane show high efficiency of CO2 fixation because of
A)
EMP pathway
done
clear
B)
TCA cycle
done
clear
C)
Hatch and Slack cycle
done
clear
D)
Calvin cycle
done
clear
View Answer play_arrow
question_answer 160) Aerobic bacteria found in hot sulphur springs are termed as
A)
halophiles
done
clear
B)
thermoacidophiles
done
clear
C)
chemolithotrophic
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 161) Cell cycle includes the sequence
A)
\[S,{{G}_{1}},{{G}_{2}},M\]
done
clear
B)
\[S,M,{{G}_{1}},{{G}_{2}}\]
done
clear
C)
\[{{G}_{1}},S,{{G}_{2}},M\]
done
clear
D)
\[M,{{G}_{1}},{{G}_{2}},S\]
done
clear
View Answer play_arrow
question_answer 162) Cell theory was proposed by
A)
Schleiden and Schwann
done
clear
B)
Watson and Crick
done
clear
C)
Darwin and Wallace
done
clear
D)
Mendel and Morgan
done
clear
View Answer play_arrow
question_answer 163) The two ends of amylose chain are designated as
A)
5 and 3 ends
done
clear
B)
N- terminal and C- terminal ends
done
clear
C)
reducing and non- reducing ends
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 164) Which of the following products of photosynthesis are used in dark reaction?
A)
ATP
done
clear
B)
NADPH + CO2
done
clear
C)
Water and oxygen
done
clear
D)
NADPH + ATP
done
clear
View Answer play_arrow
question_answer 165) Predominant form of lipid present in biomembranes
A)
triacylglycerols
done
clear
B)
cholesterol
done
clear
C)
free fatty acids
done
clear
D)
phospholipids
done
clear
View Answer play_arrow
question_answer 166) High energy phosphate bond is present in
A)
AMP
done
clear
B)
cyclic- AMP
done
clear
C)
ADP
done
clear
D)
adenosine
done
clear
View Answer play_arrow
question_answer 167) If a heterozygous tall plant is crossed with dwarf plant, what will be the ratio of dwarf plants in the progeny
A)
50%
done
clear
B)
25%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 168) Clean air is indicated by the heavy growth of
A)
mushrooms
done
clear
B)
epiphytes
done
clear
C)
lichens
done
clear
D)
grasses
done
clear
View Answer play_arrow
question_answer 169) The trace gas which is produced in rice Paddies and is associated with global warming, is
A)
methane
done
clear
B)
carbon dioxide
done
clear
C)
hydrogen sulphide
done
clear
D)
chlorine
done
clear
View Answer play_arrow
question_answer 170) Acid rain is mainly caused by the increased concentration (in the atmosphere) of
A)
CFCs and dust
done
clear
B)
\[N{{O}_{2}}\]and \[S{{O}_{2}}\]
done
clear
C)
\[N{{O}_{2}},S{{O}_{2}},N{{H}_{3}},{{H}_{2}}S\]and dust
done
clear
D)
\[N{{H}_{3}}\]and \[S{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 171) Complete genomic sequence of the following plant has been recently announced
A)
Arabidopsis thaliana
done
clear
B)
Triticum vulgare
done
clear
C)
Cucurbita pepo
done
clear
D)
Pisum sativum
done
clear
View Answer play_arrow
question_answer 172) In bacteriophages, the genetic material is
A)
RNA
done
clear
B)
DNA
done
clear
C)
mRNA
done
clear
D)
RNA or DNA
done
clear
View Answer play_arrow
question_answer 173) Duplication of DNA is called
A)
replication
done
clear
B)
transduction
done
clear
C)
transcription
done
clear
D)
translocation
done
clear
View Answer play_arrow
question_answer 174) In plasmids the R or sex factor is concerned with
A)
the transfer of genetic material from one strain to another
done
clear
B)
drug resistance
done
clear
C)
colicin production
done
clear
D)
reproduction
done
clear
View Answer play_arrow
question_answer 175) Plasmids are the molecules of
A)
DNA
done
clear
B)
RNA
done
clear
C)
Protein
done
clear
D)
DNA bound by histones
done
clear
View Answer play_arrow
question_answer 176) The bacteriophage \[\phi \times 174\]differs from other organisms in having
A)
single stranded DNA, as hereditary material
done
clear
B)
RNA as hereditary material
done
clear
C)
both DNA and RNA, as hereditary material
done
clear
D)
DNA as hereditary material
done
clear
View Answer play_arrow
question_answer 177) The codon is said to be degenerate, when
A)
the codons degenerate soon after the synthesis of polypeptide chain
done
clear
B)
one codon can code for more than one amino acid
done
clear
C)
the codon is non-functional and cannot code for any amino acid
done
clear
D)
the same amino acid can be coded by more than one codons
done
clear
View Answer play_arrow
question_answer 178) Which of the following structures is present in mitochondria?
A)
Polysomes
done
clear
B)
Dictyosomes
done
clear
C)
Quantasomes
done
clear
D)
Oxysomes
done
clear
View Answer play_arrow
question_answer 179) CO is a pollutant because it
A)
inhibits glycolysis
done
clear
B)
makes nervous system inactive
done
clear
C)
reacts with haemoglobin of blood
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 180) Which process is completed in mitochondria?
A)
Oxidative phosphorylation
done
clear
B)
Photophosphorylation
done
clear
C)
Photolysis
done
clear
D)
Matrix formation
done
clear
View Answer play_arrow
question_answer 181) Grana are present inside the
A)
mitochondria
done
clear
B)
chloroplast
done
clear
C)
endoplasmic reticulum
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 182) Which event involves in crossing over?
A)
Chromosomes are short and thick
done
clear
B)
Exchange of genetic material
done
clear
C)
Pairing of chromosomes
done
clear
D)
Addition of chromosomes
done
clear
View Answer play_arrow
question_answer 183) In mongolism a patient possesses
A)
44 chromosomes
done
clear
B)
45 chromosomes
done
clear
C)
47 chromosomes
done
clear
D)
46 chromosomes
done
clear
View Answer play_arrow
question_answer 184) The term water potential was given by
A)
Dixon and Jolly
done
clear
B)
Prof R C Sirohi
done
clear
C)
J C Bose
done
clear
D)
Slatyer and Tayler
done
clear
View Answer play_arrow
question_answer 185) Abscisic acid plays active role in
A)
dormancy of seeds
done
clear
B)
cell division
done
clear
C)
enhance senescence
done
clear
D)
shoot elongation
done
clear
View Answer play_arrow
question_answer 186) What is the name of the book written by Aristotle?
A)
Philosophic Zoologique
done
clear
B)
Systema Naturae
done
clear
C)
Historia Naturalis
done
clear
D)
Historia Animalium
done
clear
View Answer play_arrow
question_answer 187) Haemoglobin is having maximum affinity with
A)
\[N{{H}_{3}}\]
done
clear
B)
\[{{O}_{2}}\]
done
clear
C)
\[C{{O}_{2}}\]
done
clear
D)
\[CO\]
done
clear
View Answer play_arrow
question_answer 188) Humus is
A)
living organic and inorganic matter
done
clear
B)
radioactive materials
done
clear
C)
dead and decayed organic matter
done
clear
D)
only living organic matter
done
clear
View Answer play_arrow
question_answer 189) Lacteals are found in
A)
villus of intestine
done
clear
B)
kidneys
done
clear
C)
liver
done
clear
D)
lungs
done
clear
View Answer play_arrow
question_answer 190) Which one of the following exhibit concentric tube within tube plan?
A)
Annelids
done
clear
B)
Mollusca
done
clear
C)
Arthropoda
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 191) Vocal cords are situated at
A)
bronchial tube
done
clear
B)
glottis
done
clear
C)
larynx
done
clear
D)
pharynx
done
clear
View Answer play_arrow
question_answer 192) Karyotaxonomy is the modem branch of classification which is based on
A)
trinomial nomenclature
done
clear
B)
organic evolution
done
clear
C)
bands found on chromosomes
done
clear
D)
number of chromosomes
done
clear
View Answer play_arrow
question_answer 193) Under certain conditions scientists have obtained cell like structures. These are known as
A)
prebiotic soup
done
clear
B)
coacervates
done
clear
C)
protists
done
clear
D)
microbes
done
clear
View Answer play_arrow
question_answer 194) What is RQ of early stages of germination of castor seed?
A)
One
done
clear
B)
Two
done
clear
C)
Zero
done
clear
D)
Less than one
done
clear
View Answer play_arrow
question_answer 195) Which one of the following was most likely absent in free form in the primordial atmosphere at the time of origin of life?
A)
Oxygen
done
clear
B)
Ammonia
done
clear
C)
Hydrogen
done
clear
D)
Methane
done
clear
View Answer play_arrow
question_answer 196) In which book, "Binomial nomenclature" has been used for the first time?
A)
Historia Plantarum
done
clear
B)
Historia Naturalis
done
clear
C)
Systema Naturae
done
clear
D)
Histoirae Naturelle
done
clear
View Answer play_arrow
question_answer 197) The female gametophyte in angiosperm is made up of
A)
single celled
done
clear
B)
six celled
done
clear
C)
seven celled
done
clear
D)
eight celled
done
clear
View Answer play_arrow
question_answer 198) Insectivorous plants grow in
A)
calcium deficient soil
done
clear
B)
carbon deficient soil
done
clear
C)
magnesium deficient soil
done
clear
D)
nitrogen deficient soil
done
clear
View Answer play_arrow
question_answer 199) Which blood group is a universal recipient?
A)
O
done
clear
B)
AB
done
clear
C)
B
done
clear
D)
A
done
clear
View Answer play_arrow
question_answer 200) When released from ovary, the human egg contains
A)
XY- chromosomes
done
clear
B)
two X- chromosomes
done
clear
C)
one Y- chromosome
done
clear
D)
one X- chromosome
done
clear
View Answer play_arrow
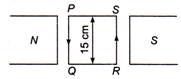

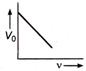
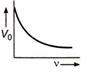
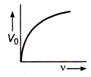
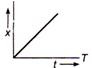
 The change in internal energy of the gas will be
The change in internal energy of the gas will be