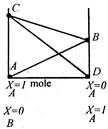
A) AB stands for the vapour pressure of component B in presence of solute A
B) CD stands for the vapour pressure of solvent A in presence of solute B
C) BC stands for the total vapour pressure in accordance with the Daltons law of partial pressures
D) all of the above
Correct Answer: D
Solution :
all of the aboveYou need to login to perform this action.
You will be redirected in
3 sec
