A) \[\frac{m}{120}\]
B) \[\frac{m}{60}\]
C) \[\frac{m}{30}\]
D) \[\frac{m}{2}\]
Correct Answer: A
Solution :
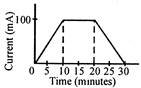
: Charge = current\[\times \]time. \[\therefore \]The area enclosed by the curve = charge The curve is a trapezium. Area of trapezium \[=\frac{1}{2}\times \]height\[\times \](sum of parallel sides) \[\therefore \]Charge\[=\frac{1}{2}\times (100\text{ }mA)\times (30+10)\text{ }min\] or Charge\[=(50\times 40\times 60)mA\times sec\] or Charge\[=\frac{50\times 40\times 60}{1000}(A\times \sec )\] \[Q=120\]coulomb. By laws of electrolysis,\[\angle Q=m\] Or \[Z=\frac{m}{Q}=\frac{m}{120}\frac{gram}{coulomb}.\]You need to login to perform this action.
You will be redirected in
3 sec
