A) square planar
B) octahedral
C) tetrahedral
D) trigonal bipyramidal with a lone pair in the equatorial position
Correct Answer: D
Solution :
:\[S{{F}_{4}}\]is irregular tetrahedral molecule resulting from\[s{{p}^{3}}d\]hybridisation of valence shell orbitals of the central S atom.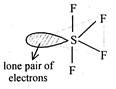
You need to login to perform this action.
You will be redirected in
3 sec
