A) \[1.5\times {{10}^{5}}\] Pa
B) \[2.5\times {{10}^{5}}\] Pa
C) \[2.1\times {{10}^{5}}\] Pa
D) \[3.5\times {{10}^{5}}\] Pa
Correct Answer: A
Solution :
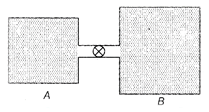
From ideal gas law For container A, \[{{n}_{1}}=\frac{{{p}_{1}}{{V}_{1}}}{R{{T}_{1}}}\] For container B, \[{{n}_{2}}=\frac{{{p}_{2}}{{V}_{2}}}{R{{T}_{2}}}\] After opening the value, x moles of gas stream from container A to container B such that both container equalize at pressure p. Number of moles in container A has changed to \[{{n}_{1}}-x,\,i.e.,\] \[({{n}_{1}}-x)=\frac{p\,.\,{{V}_{1}}}{R\,.\,{{T}_{1}}}\] \[\therefore \] \[x={{n}_{1}}=\frac{p\,.\,{{V}_{1}}}{R\,.\,{{T}_{1}}}=\frac{({{p}_{1}}-p)\,.\,{{V}_{1}}}{R\,.\,{{T}_{1}}}\] ?. (i) Number of moles in container 6 has changed to \[{{n}_{2}}+x\], therefore \[({{n}_{2}}+x)=\frac{{{p}_{2}}\,.\,{{V}_{2}}}{R\,.\,{{T}_{2}}}\] \[\therefore \] \[x=\frac{p\,.\,{{V}_{2}}}{R\,.\,{{T}_{2}}}-{{n}_{2}}=\frac{(p-{{p}_{z}}){{V}_{2}}}{R\,.\,{{T}_{2}}}\] .... (ii) Equating Eqs. (i) and (ii), we get \[\frac{({{p}_{1}}-p)\,.\,{{V}_{1}}}{R\,.\,{{T}_{1}}}=\frac{(p-{{p}_{2}})\,.\,{{V}_{2}}}{R\,.\,{{T}_{2}}}\] \[\Rightarrow \] \[({{p}_{1}}-p)=(p-{{p}_{2}})\,.\,\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\,.\,\left( \frac{{{T}_{1}}}{{{T}_{2}}} \right)\] The pressure changes in the two containers are proportional \[({{p}_{1}}-p)=(p-{{p}_{2}})\,.K\] with \[K=\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\,.\,\left( \frac{{{T}_{1}}}{{{T}_{2}}} \right)=4\,\left( \frac{300}{400} \right)=3\] \[p=\frac{{{p}_{1}}+{{p}_{2}}\,.\,K}{1+K}=\frac{5\times {{10}^{5}}+1\times {{10}^{5}}}{1+3}\] \[=\frac{6\times {{10}^{5}}}{4}=1.5\times {{10}^{5}}Pa\]You need to login to perform this action.
You will be redirected in
3 sec
