A) \[Xe{{F}_{4}}\]
B) \[BF_{4}^{-}\]
C) \[{{C}_{2}}{{H}_{4}}\]
D) \[Si{{F}_{4}}\]
Correct Answer: C
Solution :
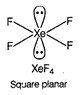
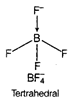
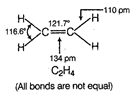
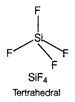 In \[{{C}_{2}}{{H}_{4}}\], carbon- carbon double bond in alkenes consists of one strong sigma bond and one weak pi (n) bond. In ethene ail the bonds are not equal. While in case of remaining species, all the bonds are equal.
In \[{{C}_{2}}{{H}_{4}}\], carbon- carbon double bond in alkenes consists of one strong sigma bond and one weak pi (n) bond. In ethene ail the bonds are not equal. While in case of remaining species, all the bonds are equal.
You need to login to perform this action.
You will be redirected in
3 sec
