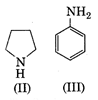
A) \[III<I<II\]
B) \[III<II<I\]
C) \[I<II<III\]
D) \[II<I<III\]
Correct Answer: A
Solution :
Basic tendency of any species is intact the tendency of accepting a proton by donating its lone pair to the proton. Among the given (III) is least basic because of the involvement of its lone pair in resonance and hence, its less availability for donation. Compound (II) is more basic than it. On the other hand compound (I) has easy availability of lone pair of electrons, so it is the most basic amine. Hence, correct order is as follows\[III<I<II\]You need to login to perform this action.
You will be redirected in
3 sec
