A) Tartaric acid, propanoic acid
B) Succinic acid, succinic anhydride
C) Maleic anhydride, maleic acid
D) Methyl malonic acid, propanoic acid
Correct Answer: D
Solution :
\[\underset{A}{\mathop{{{C}_{4}}{{H}_{6}}{{O}_{4}}}}\,\xrightarrow{\Delta }\underset{B}{\mathop{{{C}_{3}}{{H}_{6}}{{O}_{2}}}}\,\] From above equation, it is clear that \[\text{C}{{\text{O}}_{\text{2}}}\]is lost due to heating. Thus, A is a dibasic acid with two -COOH groups on same carbon atom (gem position). Thus, A is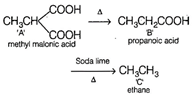 For 1 mole of C again,\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{3}}}\] \[(mol\,wt\,=30)\] \[\because \] 30 g of C occupies volume = 22.42 \[\therefore \]1 g of C with occupy volume \[\frac{22.4}{30}\] \[\simeq 0.75\,L\]
For 1 mole of C again,\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{3}}}\] \[(mol\,wt\,=30)\] \[\because \] 30 g of C occupies volume = 22.42 \[\therefore \]1 g of C with occupy volume \[\frac{22.4}{30}\] \[\simeq 0.75\,L\]
You need to login to perform this action.
You will be redirected in
3 sec
