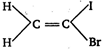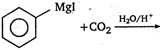question_answer 1) A body is vibrating in simple harmonic motion with amplitude of 0.06 m and frequency of 15 Hz. The maximum velocity and acceleration of the body is:
A)
9.80 m/s and \[9.03\,\times {{10}^{2}}\,m/{{s}^{2}}\]
done
clear
B)
8.90 m/s and \[8.21\times {{10}^{2}}\,m/{{s}^{2}}\]
done
clear
C)
6.82 m/s and \[7.62\times {{10}^{2}}\,m/{{s}^{2}}\]
done
clear
D)
5.65 m/s and \[5.32\,\times {{10}^{2}}\,m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 2) A motor cycle is travelling on a curved track of radius 500 m. If the coefficient of friction between tyres and road is 0.5 with \[g=10\,m/{{s}^{2}},\] what should be the maximum speed to avoid skidding?
A)
10 m/s
done
clear
B)
50 m/s
done
clear
C)
250 m/s
done
clear
D)
500m/s
done
clear
View Answer play_arrow
question_answer 3) If the equation of motion of standing wave is \[y=0.3\sin \] \[(314t-1.57x),\]the velocity of standing wave is :
A)
400 unit
done
clear
B)
250 unit
done
clear
C)
200 unit
done
clear
D)
150 unit
done
clear
View Answer play_arrow
question_answer 4) On producing the waves of frequency 1000 Hz in a Kundts tube, the total distance between 6 L successive nodes is 85 cm. Speed of sound in the gas filled in the tube is:
A)
300 m/s
done
clear
B)
350 m/s
done
clear
C)
340 m/s
done
clear
D)
330 m/s
done
clear
View Answer play_arrow
question_answer 5) A spring is vibrating with frequency under same mass. If it is cut into two equal pieces and same mass is suspended, then the new frequency will be:
A)
\[n\sqrt{2}\]
done
clear
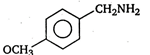
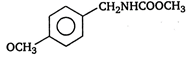
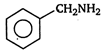
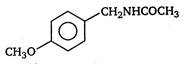
B)
\[\frac{n}{\sqrt{2}}\]
done
clear
C)
\[\frac{n}{2}\]
done
clear
D)
n
done
clear
View Answer play_arrow
question_answer 6) Diamagnetic substances are:
A)
strongly repelled by magnets
done
clear
B)
feebly repelled by magnets
done
clear
C)
feebly attracted by magnets
done
clear
D)
strongly attracted by magnets
done
clear
View Answer play_arrow
question_answer 7) The study of the effects associated with electric field at rest is known as:
A)
electrostatics
done
clear
B)
electromagnetism
done
clear
C)
magnetostatics
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 8) For driving current of 2 A for 6 min in a circuit, 1000 J of work is to be done. The emf of the source in the circuit is:
A)
1.38V
done
clear
B)
1.68V
done
clear
C)
2.03V
done
clear
D)
3.10V
done
clear
View Answer play_arrow
question_answer 9) A current carrying wire in the neighborhood produces:
A)
electric and magnetic fields
done
clear
B)
magnetic field only
done
clear
C)
no field
done
clear
D)
electric field
done
clear
View Answer play_arrow
question_answer 10) A resonance air column of length 20 cm resonates with a tuning fork of frequency 250 Hz. The speed of the air is:
A)
75 m/s
done
clear
B)
150 m/s
done
clear
C)
200 m/s
done
clear
D)
300 m/s
done
clear
View Answer play_arrow
question_answer 11) An iron rod of length 2 m and cross-sectional area of \[50\,m{{m}^{2}}\] is stretched by 0.5 mm, when a mass of 250 kg is hung from its lower end. Youngs modulus of iron rod is:
A)
\[19.6\,\times {{10}^{20}}\,N/{{m}^{2}}\]
done
clear
B)
\[19.6\,\times {{10}^{18}}\,N/{{m}^{2}}\]
done
clear
C)
\[19.6\,\times {{10}^{10}}\,N/{{m}^{2}}\]
done
clear
D)
\[19.6\,\times {{10}^{15}}\,N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 12) Frequency of infrared wave is approximately:
A)
\[{{10}^{18}}Hz\]
done
clear
B)
\[{{10}^{14}}Hz\]
done
clear
C)
\[{{10}^{9}}Hz\]
done
clear
D)
\[{{10}^{16}}Hz\]
done
clear
View Answer play_arrow
question_answer 13) A wire has resistance of 3.1\[\Omega\] at \[30{}^\circ C\] and resistance 4.5\[\Omega \] at 100°C. The temperature coefficient of resistance of the wire is :
A)
\[{{0.0012}^{0}}{{C}^{-1}}\]
done
clear
B)
\[{{0.0024}^{0}}{{C}^{-1}}\]
done
clear
C)
\[{{0.0032}^{0}}{{C}^{-1}}\]
done
clear
D)
\[{{0.0064}^{0}}{{C}^{-1}}\]
done
clear
View Answer play_arrow
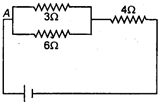
question_answer 14) If the current through 3\[\Omega \] resistor is 0.8 A, then potential drop through 4 \[\Omega \] resistor is:
A)
\[\text{1}\text{.2V}\]
done
clear
B)
\[\text{2}\text{.6V}\]
done
clear
C)
\[\text{4}\text{.8V}\]
done
clear
D)
\[\text{9}\text{.6V}\]
done
clear
View Answer play_arrow
question_answer 15) The direction of the null points on the equatorial line of a bar magnet, when the north pole of the magnet is pointing to:
A)
west
done
clear
B)
east
done
clear
C)
south
done
clear
D)
north
done
clear
View Answer play_arrow
question_answer 16) Two batteries of emf 4 V and 8 V having the internal resistance of \[1\,\Omega \] and \[2\,\Omega \] respectively are connected in circuit with a resistance of \[9\,\Omega \] as shown in a figure. The current and potential difference between the points P and Q are:
A)
\[\frac{\text{1}}{\text{12}}\text{A}\,\,\text{and}\,\text{12}\,\text{V}\]
done
clear
B)
\[\frac{\text{1}}{9}\text{A}\,\,\text{and}\,9\,\text{V}\]
done
clear
C)
\[\frac{\text{1}}{6}\text{A}\,\,\text{and}\,4\,\text{V}\]
done
clear
D)
\[\frac{\text{1}}{3}\text{A}\,\,\text{and}\,3\,\text{V}\]
done
clear
View Answer play_arrow
question_answer 17) The dimensions of gravitational constant G are:
A)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{\text{3}}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
B)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{-1}}{{\text{L}}^{2}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
C)
\[\text{ }\!\![\!\!\text{ M}{{\text{L}}^{-2}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
D)
\[\text{ }\!\![\!\!\text{ }{{\text{M}}^{-1}}{{\text{L}}^{3}}{{\text{T}}^{\text{-2}}}\text{ }\!\!]\!\!\text{ }\]
done
clear
View Answer play_arrow
question_answer 18) A 30 g bullet initially travelling at 120 m/s penetrates 12 cm into wooden block. The average resistance exerted by the wooden block is:
A)
1800 N
done
clear
B)
2000 N
done
clear
C)
2200 N
done
clear
D)
2850 N
done
clear
View Answer play_arrow
question_answer 19) Angle of dip is 90° at:
A)
equator
done
clear
B)
middle point
done
clear
C)
poles
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 20) Two magnets each of magnetic moment M are placed so as to form a cross at right angles to each other. The magnetic moment of the system will be:
A)
M
done
clear
B)
0.5 M
done
clear
C)
\[\sqrt{2}\]M
done
clear
D)
2M
done
clear
View Answer play_arrow
question_answer 21) Plate current will be maximum when:
A)
both the grid and anode are negative
done
clear
B)
both the grid and anode are positive
done
clear
C)
grid is positive and anode is negative
done
clear
D)
grid is negative and anode is positive
done
clear
View Answer play_arrow
question_answer 22) Sodium has body centered packing. Distance between two nearest atoms is \[3.7\,\overset{\text{o}}{\mathop{\text{A}}}\,\]. The lattice parameter is:
A)
\[4.9\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
B)
\[4.3\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
C)
\[3.8\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
D)
\[3.4\,\overset{\text{o}}{\mathop{\text{A}}}\,\]
done
clear
View Answer play_arrow
question_answer 23) The instrument used to measure the temperature of the source from its thermal radiation is :
A)
hydrometer
done
clear
B)
barometer
done
clear
C)
thermopile
done
clear
D)
pyrometer
done
clear
View Answer play_arrow
question_answer 24) Which of the following are not the transvene waves?
A)
Sound waves
done
clear
B)
Visible light waves
done
clear
C)
X-rays
done
clear
D)
\[\gamma \]-rays
done
clear
View Answer play_arrow
question_answer 25) The displacement x of a particle moving along a straight line at time t is given by \[x={{a}_{0}}+{{a}_{1}}t+{{a}_{2}}{{t}^{2}}\] The acceleration of the particle is:
A)
\[4{{a}_{2}}\]
done
clear
B)
\[2{{a}_{2}}\]
done
clear
C)
\[2{{a}_{1}}\]
done
clear
D)
\[{{a}_{2}}\]
done
clear
View Answer play_arrow
question_answer 26) A bomb is dropped from an aeroplane moving horizontally at constant speed. If air resistance is taken into consideration, then the bomb:
A)
falls on earth exactly below the aeroplane
done
clear
B)
falls on the earth exactly behind the aeroplane
done
clear
C)
falls on the earth ahead of the aeroplane
done
clear
D)
flies with the aeroplane
done
clear
View Answer play_arrow
question_answer 27) In a p -type semiconductor germanium is doped with:
A)
aluminium
done
clear
B)
boron
done
clear
C)
gallium
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 28) Boolean algebra is essentially based on:
A)
numbers
done
clear
B)
symbol
done
clear
C)
logic
done
clear
D)
truth
done
clear
View Answer play_arrow
question_answer 29) When a bus suddenly takes a turn, the passengers are thrown outwards because of:
A)
speed of motion
done
clear
B)
inertia of motion
done
clear
C)
acceleration of motion
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 30) The logic behind NOR gate is that which gives:
A)
high output when both inputs are high
done
clear
B)
low output when both inputs are low
done
clear
C)
high output when both inputs are low
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 31) How can the chromatic aberration be corrected?
A)
By providing different suitable curvature to its two surfaces
done
clear
B)
By combining it with another lens of opposite nature
done
clear
C)
By reducing its aperture
done
clear
D)
By providing proper polishing of its two surfaces
done
clear
View Answer play_arrow
question_answer 32) Which one of the following is not dependent on the intensity of incident photon in a photoelectric experiment?
A)
Work function of the surface
done
clear
B)
Number of photoelectrons
done
clear
C)
Stopping potential
done
clear
D)
Amount of photoelectric current
done
clear
View Answer play_arrow
question_answer 33) If red light and violet light rays are of focal lengths \[{{f}_{R}}\] and \[{{f}_{V,}}\] then which one of the following is true ?
A)
\[{{\lambda }_{R}}\le {{\lambda }_{V}}\]
done
clear
B)
\[{{\mu }_{R}}>{{\mu }_{V}}\]
done
clear
C)
\[{{\lambda }_{R}}={{\lambda }_{V}}\]
done
clear
D)
\[{{\mu }_{R}}<{{\mu }_{V}}\]
done
clear
View Answer play_arrow
question_answer 34) The large scale destruction, that would be caused due to the use of nuclear weapons is known as:
A)
neutron reproduction factor
done
clear
B)
nuclear holocaust
done
clear
C)
thermonuclear reaction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 35) If in Ramsdens eyepiece, the field lenses have focal lengths \[{{f}_{1}}\] and \[{{f}_{2}}\] respectively and separated by a distance d then :
A)
\[{{f}_{1}}=3{{f}_{2}}and\,d={{f}_{1}}+{{f}_{2}}\]
done
clear
B)
\[{{f}_{1}}={{f}_{2}}and\,d=\frac{2}{3}{{f}_{1}}\]
done
clear
C)
\[{{f}_{1}}=\frac{2}{3}{{f}_{2}}and\,d=\frac{2}{3}{{f}_{1}}\]
done
clear
D)
\[{{f}_{1}}={{f}_{2}}and\,d={{f}_{1}}+{{f}_{2}}\]
done
clear
View Answer play_arrow
question_answer 36) Huygens wave theory of light could not explain:
A)
photoelectric effect
done
clear
B)
polarization
done
clear
C)
diffraction
done
clear
D)
interference
done
clear
View Answer play_arrow
question_answer 37) The rain drops are spherical in shape due to:
A)
residual pressure
done
clear
B)
thrust on drop
done
clear
C)
surface tension
done
clear
D)
viscosity
done
clear
View Answer play_arrow
question_answer 38) The work done in pulling up a block of wood weighing 2 kN for a length of 10 m on a smooth plane inclined at an angle of 15° with the horizontal is:
A)
9.82 kJ
done
clear
B)
8.91 kJ
done
clear
C)
5.17 kJ
done
clear
D)
4.36 kJ
done
clear
View Answer play_arrow
question_answer 39) The thermions are:
A)
positrons
done
clear
B)
photons
done
clear
C)
electrons
done
clear
D)
protons
done
clear
View Answer play_arrow
question_answer 40) The internal resistance of cell of emf 2 V is\[0.1\,\Omega .\] It is connected to a resistance of 3.9 \[\Omega .\] The voltage across the cell is:
A)
2.71 V
done
clear
B)
1.95 V
done
clear
C)
1.68 V
done
clear
D)
0.52 V
done
clear
View Answer play_arrow
question_answer 41) The earth of mass \[6\times {{10}^{24}}\] kg revolves around the sun with an angular velocity of \[2\times {{10}^{-7}}rad/s\]. In a circular orbit of radius \[1.5\times {{10}^{8}}\,km,\] the force exerted by the sun, on the earth is:
A)
\[\text{27 }\!\!\times\!\!\text{ 1}{{\text{0}}^{\text{39}}}\text{N}\]
done
clear
B)
\[\text{36 }\!\!\times\!\!\text{ 1}{{\text{0}}^{21}}\text{N}\]
done
clear
C)
\[\text{18 }\!\!\times\!\!\text{ 1}{{\text{0}}^{25}}\text{N}\]
done
clear
D)
\[\text{6 }\!\!\times\!\!\text{ 1}{{\text{0}}^{19}}\text{N}\]
done
clear
View Answer play_arrow
question_answer 42) A stone is thrown with an initial speed of 4.9 m/s from a bridge in vertically upward direction. It falls down in water after 2 s. The height of the bridge is:
A)
24.7 m
done
clear
B)
19.8 m
done
clear
C)
9.8 m
done
clear
D)
4.9 m
done
clear
View Answer play_arrow
question_answer 43) A ball of mass 150g moving with an acceleration \[20\,m/{{s}^{2}}\] is hit by a force, which acts, on it for 0.1 s. The impulsive force is:
A)
1.2N-s
done
clear
B)
0.3 N-s
done
clear
C)
0.1 N-s
done
clear
D)
0.5 N-s
done
clear
View Answer play_arrow
question_answer 44) If the heat of 110 J is added to a gaseous system, whose internal energy is 40 J, then the amount of external work done is:
A)
80 J
done
clear
B)
70 J
done
clear
C)
115 J
done
clear
D)
140 J
done
clear
View Answer play_arrow
question_answer 45) The substance in which the magnetic moment of a single atom is not zero, is called as:
A)
ferrimagnetism
done
clear
B)
paramagnerism
done
clear
C)
ferromagnetism
done
clear
D)
diamagnetism
done
clear
View Answer play_arrow
question_answer 46) The luminous efficiency of a lamp is 4 lumen/W and its luminous intensity is 30 Cd The power of lamp is:
A)
60 W
done
clear
B)
78 W
done
clear
C)
94 W
done
clear
D)
136 W
done
clear
View Answer play_arrow
question_answer 47) The transfer ratio \[\] of a transistor is 50. The input resistance of the transistor when used in the common-emitter configuration is 1 \[\Omega \]. The peak value of the collector AC current for an AC input voltage of 0.01 V, is:
A)
500 \[\text{A}\]
done
clear
B)
0.25\[\text{A}\]
done
clear
C)
0.01 \[\text{A}\]
done
clear
D)
100 \[\text{A}\]
done
clear
View Answer play_arrow
question_answer 48) Two vectors \[\mathbf{\vec{A}}\] and \[\mathbf{\vec{B}}\] are such that\[\mathbf{\vec{A}}\,\mathbf{\vec{B}}=\mathbf{\vec{C}}\] and \[{{A}^{2}}+{{B}^{2}}={{C}^{2}}.\] If \[\theta \] is the angle between \[\mathbf{\vec{A}}\] and \[\,\mathbf{\vec{B}}\] then correct statement is:
A)
\[\theta =\pi \]
done
clear
B)
\[\theta =\frac{2\pi }{3}\]
done
clear
C)
\[\theta =0\]
done
clear
D)
\[\theta =\frac{\pi }{2}\]
done
clear
View Answer play_arrow
question_answer 49) A rough vertical board has an acceleration \[a\] along the horizontal, so that a block of mass M pressing against it does not fall. The coefficient of friction between block and the board is:
A)
\[>\frac{a}{g}\]
done
clear
B)
\[<\frac{g}{a}\]
done
clear
C)
\[=\frac{a}{g}\]
done
clear
D)
\[>\frac{g}{a}\]
done
clear
View Answer play_arrow
question_answer 50) An aeroplane is moving with a horizontal velocity u at a height h. The velocity of packet dropped from it on the earths surface will be:
A)
\[\sqrt{{{u}^{2}}-2gh}\]
done
clear
B)
\[2gh\]
done
clear
C)
\[\sqrt{2gh}\]
done
clear
D)
\[\sqrt{{{u}^{2}}+2gh}\]
done
clear
View Answer play_arrow
question_answer 51) Which of the following having highest number of molecules?
A)
\[16g\,{{O}_{2}}\]
done
clear
B)
\[14\,g\,{{N}_{2}}\]
done
clear
C)
\[2g\,{{H}_{2}}\]
done
clear
D)
\[6\,g\,{{I}_{2}}\]
done
clear
View Answer play_arrow
question_answer 52) The size of isoelectronic ions depends on:
A)
ionization energy
done
clear
B)
nuclear charge
done
clear
C)
ionic radius
done
clear
D)
covalent radius
done
clear
View Answer play_arrow
question_answer 53) At constant temperature the pressure of a gas is increased to three times, then its volume becomes:
A)
\[\frac{V}{3}\]
done
clear
B)
\[\frac{2}{3}V\]
done
clear
C)
\[3\,V\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 54) Find the temperature of hydrogen gas which has the same velocity as that of oxygen at a temperature of \[0{{\,}^{o}}C:\]
A)
\[273\div 16\text{ }K\]
done
clear
B)
\[~273\times 8\,K\]
done
clear
C)
\[273\div 32K\]
done
clear
D)
\[273\times 4\,K\]
done
clear
View Answer play_arrow
question_answer 55) What is the bond order in case of\[O_{2}^{+}\]?
A)
2.5
done
clear
B)
2
done
clear
C)
1.5
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 56) Lindlars catalyst is:
A)
\[Pd\] supported over \[\text{BaC}{{\text{O}}_{\text{3}}}\]
done
clear
B)
Hg supported over \[PbS{{O}_{4}}\]
done
clear
C)
Ni supported over\[~CuS{{O}_{4}}\]
done
clear
D)
Ni supported over \[CdS{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 57) In\[s{{p}^{3}}d\]-hybridisation, the \[d-\]orbital that participate in hybridisation is:
A)
\[dxy\]
done
clear
B)
\[dxz\]
done
clear
C)
\[d{{x}^{2}}-{{y}^{2}}\]
done
clear
D)
\[d{{z}^{2}}\]
done
clear
View Answer play_arrow
question_answer 58) Which of the following is correct in the reaction? \[{{H}_{2}}(g)+{{I}_{2}}(g)\xrightarrow{{}}2HI(g)\]
A)
\[{{K}_{p}}={{K}_{c}}\]
done
clear
B)
\[{{K}_{p}}>{{K}_{c}}\]
done
clear
C)
\[{{K}_{p}}<{{K}_{c}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 59) A \[CaC{{O}_{3}}\]sample contains \[3.01\times {{10}^{23}}\] ions of \[\overset{2+}{\mathop{Ca}}\,\]and \[C{{O}_{3}}^{2-}.\]The mass of sample is:
A)
40
done
clear
B)
50
done
clear
C)
60
done
clear
D)
70
done
clear
View Answer play_arrow
question_answer 60) The increasing order for the value of charge/mass for electron (e), proton (p), neutron (n) and alpha particle \[(\alpha )\] is:
A)
\[e<p<n<a\]
done
clear
B)
\[~n<p<e<\alpha \]
done
clear
C)
\[n<p<\alpha <e\]
done
clear
D)
\[n<\alpha <p<e\]
done
clear
View Answer play_arrow
question_answer 61) The bond order of \[{{O}_{2}}^{-}\]is:
A)
1
done
clear
B)
zero
done
clear
C)
1.5
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 62) d and \[l\]tartaric adds are:
A)
enantiomers
done
clear
B)
tautomers
done
clear
C)
mesomers
done
clear
D)
diastereomers
done
clear
View Answer play_arrow
question_answer 63) In the reaction, \[2C(s)+{{O}_{2}}(g)2CO\,(g)\] the partial pressure of CO and \[{{O}_{2}}\]is 8 atm and 4 atm respectively, then find its equilibrium constant:
A)
16
done
clear
B)
24
done
clear
C)
8
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 64) Which is the correct electronic configuration of Cr (chromium)?
A)
\[1{{s}^{2}}\text{ }2{{s}^{2}}\text{ }2{{p}^{6}}\text{ }3{{s}^{2}}\text{ }3{{p}^{6}}\text{ }4{{s}^{1}}\text{ }3{{d}^{5}}\]
done
clear
B)
\[1{{s}^{2}}\text{ }2{{s}^{2}}\text{ }2{{p}^{6}}\text{ }3{{s}^{2}}\text{ }3{{p}^{6}}\text{ }4{{s}^{2}}\text{ }3{{d}^{4}}\]
done
clear
C)
\[1{{s}^{2}}\text{ }2{{s}^{2}}\text{ }2{{p}^{6}}\text{ }3{{s}^{2}}\text{ }3{{p}^{6}}\text{ }4{{s}^{2}}\text{ }3{{d}^{6}}\]
done
clear
D)
\[1{{s}^{2}}\text{ }2{{s}^{2}}\text{ }2{{p}^{6}}\text{ }3{{s}^{2}}\text{ }3{{p}^{6}}\text{ }4{{s}^{1}}\text{ }3{{d}^{8}}\]
done
clear
View Answer play_arrow
question_answer 65) If heat of neutralization of \[\text{C}{{\text{H}}_{\text{3}}}\text{COOH}\]and \[NaOH\] is \[-\,50.6\text{ kJ}\]equivalent and the heat of neutralization of \[\text{N}{{\text{H}}_{\text{4}}}\text{OH}\]and \[\text{HCl}\]is \[-51.4\text{ kJ}\]equivalent then the heat of neutralization of \[\text{C}{{\text{H}}_{\text{3}}}\text{COOH}\]and \[\text{N}{{\text{H}}_{\text{4}}}\text{OH}\]is:
A)
\[\text{ }\!\!~\!\!\text{ 102}\,\text{k J}\]
done
clear
B)
\[-0.8\text{ kJ}\]
done
clear
C)
\[0.8\text{ kJ}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 66) At \[25{{\,}^{o}}C\,{{K}_{a}}\]for\[~C{{H}_{3}}COOH\] is\[~1.8\times {{10}^{-5}}\]and \[{{K}_{b}}\]for \[\text{N}{{\text{H}}_{\text{4}}}\text{OH}\]is also \[\text{1}\text{.8}\times {{10}^{-5}}.\]The nature of aqueous solution of \[\text{C}{{\text{H}}_{\text{3}}}\text{COON}{{\text{H}}_{\text{4}}}\]is:
A)
neutral
done
clear
B)
basic
done
clear
C)
acidic
done
clear
D)
amphoteric
done
clear
View Answer play_arrow
question_answer 67) On bombarding \[{{\,}_{7}}{{N}^{14}}\] with \[\alpha -\]particles, the nuclei of the product formed after the release of a proton is:
A)
\[{{\,}_{8}}{{O}^{17}}\]
done
clear
B)
\[{{\,}_{8}}{{O}^{18}}\]
done
clear
C)
\[{{\,}_{9}}{{F}^{7}}\]
done
clear
D)
\[{{\,}_{9}}{{F}^{18}}\]
done
clear
View Answer play_arrow
question_answer 68) Coordination number and oxidation number of Cr in \[{{K}_{3}}Cr{{({{C}_{2}}{{O}_{4}})}_{3}}\]are respectively:
A)
6 and \[+\,3\]
done
clear
B)
4 and\[-\,2\]
done
clear
C)
3 and 0
done
clear
D)
3 and\[+\,3\]
done
clear
View Answer play_arrow
question_answer 69) Thermite is a mixture of:
A)
\[MgO+Al\]
done
clear
B)
\[F{{e}_{3}}{{O}_{4}}+Al\]
done
clear
C)
\[Zn+CaC{{O}_{3}}\]
done
clear
D)
\[Zn+{{P}_{2}}{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 70) Geometrical isomerism is shown by:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 71) The oxidation state of sulphur in \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{:}\]
A)
\[+\,6\]
done
clear
B)
\[~+\,4\]
done
clear
C)
\[~+\,2\]
done
clear
D)
\[+\,3\]
done
clear
View Answer play_arrow
question_answer 72) For detection of sulphur in an organic compound, sodium nitroprusside is added to the Lassaignes filtrate the ppt. obtained is:
A)
purple colour
done
clear
B)
black colour
done
clear
C)
blood-red colour
done
clear
D)
white colour
done
clear
View Answer play_arrow
question_answer 73) Which of the following is correct for \[{{\,}^{60}}Co\xrightarrow{-{{\beta }^{-}}}?\]
A)
\[{{\,}^{61}}Co\]
done
clear
B)
\[{{\,}^{60}}Ni\]
done
clear
C)
\[{{\,}^{59}}Ni\]
done
clear
D)
\[{{\,}^{60}}Cu\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following is not linear?
A)
\[BeC{{l}_{2}}\]
done
clear
B)
HCN
done
clear
C)
\[~ZnC{{l}_{2}}\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following compound is optically active?
A)
\[C{{H}_{3}}C{{H}_{2}}COOH\]
done
clear
B)
\[C{{H}_{3}}CHOHCOOH\]
done
clear
C)
\[HOOC.C{{H}_{2}}.COOH\]
done
clear
D)
\[C{{H}_{3}}.CO.COOH\]
done
clear
View Answer play_arrow
question_answer 76) The boiling point of phenols are higher than the hydrocarbons of comparable masses due to:
A)
more polarizing power
done
clear
B)
presence of hydrogen bonding
done
clear
C)
resonance stabilization
done
clear
D)
acidic character
done
clear
View Answer play_arrow
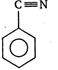
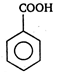
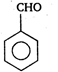
question_answer 77)
The product obtained is:
A)
benzoic acid
done
clear
B)
benzaldehyde
done
clear
C)
salicylaldehyde
done
clear
D)
salicylic acid
done
clear
View Answer play_arrow
question_answer 78) The reduction of aldehyde and .ketones to the corresponding hydrocarbons with amalgamated zinc and concentrated\[\text{HCl}\] is called:
A)
Wolff-Kishner reduction
done
clear
B)
Clemmensen reduction
done
clear
C)
Coupling reduction
done
clear
D)
Cross- Cannizaro reaction
done
clear
View Answer play_arrow
question_answer 79) The enthalpy and entropy change for a reaction is \[-2.\text{ }5\times {{10}^{3}}\,\text{cal}\] cat and \[7.4\text{ cal de}{{\text{g}}^{-1}}\]respectively. At 298 K the reaction is:
A)
reversible
done
clear
B)
irreversible
done
clear
C)
spontaneous
done
clear
D)
non-spontaneous
done
clear
View Answer play_arrow
question_answer 80) The energy of an electron in the first Bohr orbit is \[-\text{13}\text{.6 eV,}\] then find the energy of \[\text{H}{{\text{e}}^{\text{+}}}\]in the same Bohr orbit:
A)
\[27.2\,eV\]
done
clear
B)
\[-27.2\,\,eV\]
done
clear
C)
\[54.4\,eV\]
done
clear
D)
\[54.4\,eV\]
done
clear
View Answer play_arrow
question_answer 81) Aldehydes and ketones can be distinguished by:
A)
Tollens reagent
done
clear
B)
2, 4 DNP test
done
clear
C)
Molisch test
done
clear
D)
Mullikans test
done
clear
View Answer play_arrow
question_answer 82) The electrical conductance is shown by:
A)
sodium
done
clear
B)
diamond
done
clear
C)
graphite
done
clear
D)
potassium
done
clear
View Answer play_arrow
question_answer 83) The largest number of molecules are present in:
A)
\[5g\,N{{H}_{3}}\]
done
clear
B)
\[11\,g\,C{{O}_{2}}\]
done
clear
C)
\[8\,gS{{O}_{2}}\]
done
clear
D)
\[~4\,g\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 84) The splitting of spectral lines under the influence of magnetic field is known as:
A)
Zeeman effect
done
clear
B)
photoelectric effect
done
clear
C)
Stark effect
done
clear
D)
electromagnetic effect
done
clear
View Answer play_arrow
question_answer 85) Write the product: \[\underset{C{{H}_{2}}OH}{\mathop{\underset{|}{\mathop{C{{H}_{2}}OH}}\,}}\,\xrightarrow{HI{{O}_{4}}}\]
A)
formaldehyde
done
clear
B)
acetaldehyde
done
clear
C)
formic acid
done
clear
D)
acetic acid
done
clear
View Answer play_arrow
question_answer 86) lonization energy of hydrogen is:
A)
slightly higher than chlorine
done
clear
B)
much higher than chlorine
done
clear
C)
lesser than chlorine
done
clear
D)
equal to chlorine
done
clear
View Answer play_arrow
question_answer 87) In LPG gas the main constituent of the gas is:
A)
ethane
done
clear
B)
methane
done
clear
C)
butane
done
clear
D)
propane
done
clear
View Answer play_arrow
question_answer 88) Which plays a major role in the formation of complex compound?
A)
Transition metal
done
clear
B)
Lanthanides and actinides
done
clear
C)
Representative elements
done
clear
D)
p-block element
done
clear
View Answer play_arrow
question_answer 89) The conjugate base of \[{{\text{H}}_{\text{2}}}\text{PO}_{4}^{-}\]is:
A)
\[{{H}_{3}}P{{O}_{4}}\]
done
clear
B)
\[HP{{O}_{4}}^{2-}\]
done
clear
C)
\[P{{O}_{4}}^{3-}\]
done
clear
D)
\[{{H}_{2}}PO{{ }_{3}}^{-}\]
done
clear
View Answer play_arrow
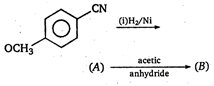
question_answer 90)
The product obtained from:
A)
done
clear
B)
done
clear
C)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 91) Benzaldehyde on treatment with ethanolic KCN produce:
A)
\[{{C}_{6}}{{H}_{5}}COCO{{C}_{6}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{6}}{{H}_{5}}CHOHCN\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}CHOHCOOH\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}CHOHCO{{C}_{6}}{{H}_{5}}\]
done
clear
View Answer play_arrow
question_answer 92) When aniline is warm with \[\text{CHC}{{\text{l}}_{\text{3}}}\]and alc. KOH, it forms a compound which having offensive smell, the compound formed is:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 93) The formation of large number of compounds of carbon is due to its:
A)
non-metallic nature
done
clear
B)
catenation character
done
clear
C)
high ionization potential
done
clear
D)
four valency
done
clear
View Answer play_arrow
question_answer 94) Orthophosphoric acid is:
A)
monobasic
done
clear
B)
dibasic
done
clear
C)
tribasic
done
clear
D)
tetrabasic
done
clear
View Answer play_arrow
question_answer 95) The volume strength of \[\text{1}\text{.5}\,\text{(N)}\,{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\]solution is:
A)
4.8
done
clear
B)
8.4
done
clear
C)
3
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 96) A gas has a vapour density 11.2. The volume occupied by 1 g of gas at NTP is:
A)
1 L
done
clear
B)
11.2 L
done
clear
C)
22.4 L
done
clear
D)
unpredictable
done
clear
View Answer play_arrow
question_answer 97) What is the rate of a reaction in a first order reaction, if its half life period is 693 s and its concentration is\[\text{2 mol/L}\]?
A)
100
done
clear
B)
0.002
done
clear
C)
0.02
done
clear
D)
200
done
clear
View Answer play_arrow
question_answer 98)
Write the product formed in the reaction:
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) The most radioactive element is:
A)
radium
done
clear
B)
uranium
done
clear
C)
polonium
done
clear
D)
thorium
done
clear
View Answer play_arrow
question_answer 100) A compound contains 38.8% C, 16% H and 45.2% N. The empirical formula of the compound will be:
A)
\[C{{H}_{5}}N\]
done
clear
B)
\[{{C}_{2}}{{H}_{5}}N\]
done
clear
C)
\[CHN\]
done
clear
D)
\[C{{H}_{10}}{{N}_{2}}\]
done
clear
View Answer play_arrow
question_answer 101) The minimum value of n for\[{{\left( \frac{1+i}{1-i} \right)}^{n}}=1,\] where \[i=\sqrt{-1}\] is:
A)
2
done
clear
B)
4
done
clear
C)
6
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 102) Find the value of \[\sqrt{6+\sqrt{6}+\sqrt{6...\infty }}:\]
A)
2
done
clear
B)
3
done
clear
C)
\[-\,4\]
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 103) If \[\arg \,\left( \frac{z-1}{z+1} \right)=\frac{\pi }{3},\]then given locus represent a:
A)
straight line
done
clear
B)
circle
done
clear
C)
parabola
done
clear
D)
ellipse
done
clear
View Answer play_arrow
question_answer 104) If \[{{z}_{k}}=\cos \frac{\theta }{{{2}^{k}}}+i\sin \frac{\theta }{{{2}^{k}}}\]where\[k=1,2,3,....,\]and \[\theta =2n\pi ,n\in \operatorname{I},\]then \[{{z}_{1}},{{z}_{2}},{{z}_{3}},....\infty \]is equal to
A)
0
done
clear
B)
1
done
clear
C)
\[-1\]
done
clear
D)
\[i\]
done
clear
View Answer play_arrow
question_answer 105) \[{{(sin\theta +cos\theta )}^{4}}\]is equal to:
A)
\[\sin 4\,\theta +i\cos 4\,\theta \]
done
clear
B)
\[\sin 4\,\theta -i\sin 2\theta \]
done
clear
C)
0
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 106) The angle of elevation of the top of an incomplete vertical pillar at a horizontal distance of 100 m from its base is \[\text{4}{{\text{5}}^{\text{o}}}\text{.}\] If the angle of elevation of the top of the complete pillar at the same point is to be 60°, then the height of the incomplete pillar is to be increased by:
A)
\[\text{50}\sqrt{3}\,m\]
done
clear
B)
\[100\,m\]
done
clear
C)
\[100(\sqrt{3}-1)\,m\]
done
clear
D)
\[100(\sqrt{3}+1)\,m\]
done
clear
View Answer play_arrow
question_answer 107) If \[{{x}^{2}}+{{y}^{2}}=25,xy=12,\]then \[x\]is equal to:
A)
\[3,4\]
done
clear
B)
\[3,-3\]
done
clear
C)
\[3,4,-3,-4\]
done
clear
D)
\[~-3,-3\]
done
clear
View Answer play_arrow
question_answer 108) If \[b+c,c+a,a+b\]are in HP, then \[{{a}^{2}},{{b}^{2}},{{c}^{2}}\]are in:
A)
AP
done
clear
B)
HP
done
clear
C)
GP
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 109) Series\[\frac{1}{1!(n-1)}+\frac{1}{3!(n-3)!}+\frac{1}{5!(n-5)!}+...\]is equal to:
A)
\[\frac{{{2}^{n}}}{n!}\]
done
clear
B)
\[\frac{{{2}^{n-1}}}{n!}\]
done
clear
C)
0
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 110) The number of terms in the expansion of \[{{(a+b+c)}^{n}}\]will be:
A)
\[n+1\]
done
clear
B)
\[n+3\]
done
clear
C)
\[\frac{(n+1)\,(n+2)}{2}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 111) If the equation \[{{x}^{2}}-4x+2(m+1)=0\]has real roots, then the value of \[m\] lies in the interval:
A)
\[-2\le m\le 1\]
done
clear
B)
\[-1\le m\le 1\]
done
clear
C)
\[2<m<3\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 112) If \[{{x}^{2}}+ax+\beta =0\]and \[{{x}^{2}}+px+q=0\] has a common roof, then the common root is:
A)
\[\frac{q+\beta }{a+p}\]
done
clear
B)
\[\frac{q-\beta }{a+p}\]
done
clear
C)
\[\frac{q-\beta }{a-p}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 113) Equation of the pair of tangents drawn from the origin to the circle \[{{x}^{2}}+{{y}^{2}}+2gx+2fy+c=0\] is:
A)
\[gx+fy=c({{x}^{2}}+{{y}^{2}})\]
done
clear
B)
\[{{(gx+fy)}^{2}}={{x}^{2}}+{{y}^{2}}\]
done
clear
C)
\[{{(gx+fy)}^{2}}={{c}^{2}}({{x}^{2}}+{{y}^{2}})\]
done
clear
D)
\[{{(gx+fy)}^{2}}=c({{x}^{2}}+{{y}^{2}})\]
done
clear
View Answer play_arrow
question_answer 114) The length of transverse axis of the hyperbola \[3{{x}^{2}}-4{{y}^{2}}=32\]is:
A)
\[\frac{8\sqrt{2}}{\sqrt{3}}\]
done
clear
B)
\[\frac{16\sqrt{2}}{\sqrt{3}}\]
done
clear
C)
\[\frac{3}{32}\]
done
clear
D)
\[\frac{64}{3}\]
done
clear
View Answer play_arrow
question_answer 115) The value of coefficient which is independent from \[x\] in\[{{\left( 2x-\frac{3}{{{x}^{2}}} \right)}^{6}},\]is :
A)
\[~-\,2916\]
done
clear
B)
4860
done
clear
C)
2160
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 116) The coefficient of \[{{x}^{4}}\] in the expansion of \[{{(1+x+{{x}^{2}}+{{x}^{3}})}^{n}},\] is:
A)
\[{{\,}^{n}}{{C}_{4}}\]
done
clear
B)
\[{{\,}^{n}}{{C}_{4}}+{{\,}^{n}}{{C}_{2}}\]
done
clear
C)
\[{{\,}^{n}}{{C}_{4}}+{{\,}^{n}}{{C}_{4}}+{{\,}^{n}}{{C}_{2}}\]
done
clear
D)
\[{{\,}^{n}}{{C}_{4}}+{{\,}^{n}}{{C}_{2}}^{n}{{C}_{1}}+{{\,}^{n}}{{C}_{2}}\]
done
clear
View Answer play_arrow
question_answer 117) In \[\Delta \,ABC,\frac{\cos 2A}{{{a}^{2}}}-\frac{\cos 2B}{{{b}^{2}}}\] is equal to:
A)
\[{{c}^{2}}/{{a}^{2}}{{b}^{2}}\]
done
clear
B)
\[\frac{1}{{{a}^{2}}}-\frac{1}{{{b}^{2}}}\]
done
clear
C)
\[\frac{1}{ab}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 118) If \[A=\left[ \begin{matrix} \cos x & \sin x \\ -\sin x & \cos x \\ \end{matrix} \right],\]then the inverse of A is where n = 1, 2, 3, ?:
A)
\[\left[ \begin{matrix} {{\cos }^{n}}x & {{\sin }^{n}}x \\ -{{\sin }^{n}}x & {{\cos }^{n}}x \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} {{\cos }^{2}}nx & {{\sin }^{2}}nx \\ -{{\sin }^{2}}nx & {{\cos }^{2}}nx \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} \cos nx & \sin nx \\ -\sin \,nx & \cos \,nx \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 1 & 0 \\ 0 & 1 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 119) If \[{{A}^{2}}-A+I=0,\]then the inverse of A is:
A)
\[{{A}^{-1}}\]
done
clear
B)
\[A+I\]
done
clear
C)
\[I-A\]
done
clear
D)
\[A-I\]
done
clear
View Answer play_arrow
question_answer 120) Let \[A=\left[ \begin{matrix} 1 & 2 \\ 0 & 1 \\ \end{matrix} \right],\] then \[{{A}^{n}}\]is equal to:
A)
\[\left[ \begin{matrix} 1 & 2n \\ 0 & 2 \\ \end{matrix} \right]\]
done
clear
B)
\[\left[ \begin{matrix} 2 & n \\ 0 & 1 \\ \end{matrix} \right]\]
done
clear
C)
\[\left[ \begin{matrix} 1 & 2n \\ 0 & 1 \\ \end{matrix} \right]\]
done
clear
D)
\[\left[ \begin{matrix} 1 & n \\ 0 & 2 \\ \end{matrix} \right]\]
done
clear
View Answer play_arrow
question_answer 121) In triangle \[ABC,\angle A={{90}^{o}}\]and \[AB=AC\]and the coordinate of B and C are (-3, 6) and (1,2) respectively, then the area of triangle is:
A)
4 sq unit
done
clear
B)
\[4\sqrt{2}\,\]sq unit
done
clear
C)
8 sq unit
done
clear
D)
\[\left( -\frac{1}{5},-\frac{22}{5} \right)\]
done
clear
View Answer play_arrow
question_answer 122) If \[x+2y+1=0,\] then reflection point of \[(3,2)\] is:
A)
\[(1,4)\]
done
clear
B)
\[(1,-\,4)\]
done
clear
C)
\[(4,1)\]
done
clear
D)
\[\left( -\frac{1}{5},-\frac{22}{5} \right)\]
done
clear
View Answer play_arrow
question_answer 123) Re \[{{\left( \frac{1+i}{3-i} \right)}^{2}}\] is equal to:
A)
\[-1/5\]
done
clear
B)
\[~1/5\]
done
clear
C)
\[~1/10\]
done
clear
D)
\[~-1/10\]
done
clear
View Answer play_arrow
question_answer 124) Which term of the series \[3+8+13+18+....\] is 498:
A)
95th
done
clear
B)
100th
done
clear
C)
102th
done
clear
D)
101th
done
clear
View Answer play_arrow
question_answer 125) If one root of \[5{{x}^{2}}+13x+k=0\] is reciprocal of the other, then k is equal to:
A)
0
done
clear
B)
5
done
clear
C)
1/6
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 126) The point having position vectors \[2\hat{i}+3\hat{j}+4\hat{k},3\hat{i}+4\hat{j}+2\hat{k},4\hat{i}+2\hat{j}+3\hat{k}\] are the vertices of:
A)
right angled triangle
done
clear
B)
isosceles triangle
done
clear
C)
equilateral triangle
done
clear
D)
collinear
done
clear
View Answer play_arrow
question_answer 127) If a polygon has 44 diagonals, then the number of its side are:
A)
7
done
clear
B)
11
done
clear
C)
8
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 128) \[{{\sin }^{-1}}\frac{3}{5}+{{\tan }^{-1}}\frac{1}{7}\]is equal to:
A)
\[\pi /2\]
done
clear
B)
\[{{\cos }^{-1}}4/5\]
done
clear
C)
\[\pi \]
done
clear
D)
\[\pi /4\]
done
clear
View Answer play_arrow
question_answer 129) Let p be the proposition that mathematics is interesting and let be the proposition that mathematics is difficult, then the symbol \[p\wedge q\] means:
A)
mathematics is interesting, implies that mathematics is difficult
done
clear
B)
mathematics is interesting implies and is implied by mathematics is difficult
done
clear
C)
mathematics is interesting and mathematics is difficult
done
clear
D)
mathematics is interesting or mathematics is difficult
done
clear
View Answer play_arrow
question_answer 130) The statement \[p\vee \tilde{\ }p\]is:
A)
tautology
done
clear
B)
contradiction
done
clear
C)
neither a tautology nor a contradiction
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 131) Which of the following function is periodic with period\[\pi \]?
A)
\[f(x)=x\cos x\]
done
clear
B)
\[f(x)=|\cos x|\]
done
clear
C)
\[f(x)=\sin x\]
done
clear
D)
\[f(x)=[x+\pi ]\] Where \[[x]\] means the greater integer not greater than \[x.\]
done
clear
View Answer play_arrow
question_answer 132) \[\frac{{{e}^{2}}+1}{2e}\]is equal to:
A)
\[1+\frac{1}{2!}+\frac{1}{4!}+\frac{1}{6!}+....\infty \]
done
clear
B)
0
done
clear
C)
1
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 133) \[\underset{x\to 0}{\mathop{\lim }}\,\frac{x{{\sin }^{-1}}}{\sin {{x}^{2}}}\]is equal to:
A)
0
done
clear
B)
1
done
clear
C)
2
done
clear
D)
\[\infty \]
done
clear
View Answer play_arrow
question_answer 134) If\[y={{\sin }^{-1}}(\cos x),\] then \[\frac{dy}{dx}\]is equal to:
A)
\[\frac{1}{\sin x}\]
done
clear
B)
\[{{\cos }^{-1}}x\]
done
clear
C)
\[-1\]
done
clear
D)
\[\frac{1}{2}\]
done
clear
View Answer play_arrow
question_answer 135) If \[{{e}^{x}}\sin \,y-{{e}^{y}}\cos x=1,\] then \[\frac{dy}{dx}\]is equal to:
A)
\[\frac{{{e}^{x}}\sin y+{{e}^{y}}\sin x}{{{e}^{y}}\cos x-{{e}^{x}}\cos y}\]
done
clear
B)
\[\frac{{{e}^{x}}\sin x+{{e}^{y}}\sin y}{{{e}^{y}}\cos x-{{e}^{x}}\cos y}\]
done
clear
C)
\[\frac{{{e}^{x}}\sin y-{{e}^{y}}\sin x}{{{e}^{y}}\cos x-{{e}^{x}}\cos y}\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 136) If \[y=\cos \,t\] and\[x=\sin t,\], then \[\frac{{{d}^{2}}y}{d{{x}^{2}}}\]is equal to:
A)
\[\frac{y}{x}\]
done
clear
B)
\[\frac{x}{y}\]
done
clear
C)
0
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 137) The tangent to the curve \[y={{e}^{2x}}\]at the point (0, 1) meet the axis at:
A)
\[(0,4)\]
done
clear
B)
\[(2,0)\]
done
clear
C)
\[(-1/2,0)\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 138) \[{{\log }_{e}}x\]is equal to:
A)
\[(x-1)-\frac{{{(x-1)}^{2}}}{2}+\frac{{{(x-1)}^{3}}}{3}-....\infty \]
done
clear
B)
0
done
clear
C)
1
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 139) \[\int_{{}}^{{}}{\frac{x{{e}^{x}}}{{{(x+1)}^{2}}}dx}\]is equal to:
A)
\[\frac{{{e}^{x}}}{x+1}+c\]
done
clear
B)
\[\frac{{{e}^{x}}}{{{(x+1)}^{2}}}+c\]
done
clear
C)
\[\frac{{{e}^{x}}}{{{(x+1)}^{3}}}+c\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 140) \[\int_{{}}^{{}}{\frac{\tan x}{\sec x+\tan x}dx}\]is equal to:
A)
\[x+\sec x+\tan x+c\]
done
clear
B)
\[x-\sec x+\tan x+c\]
done
clear
C)
\[x-\tan x+\sec x+c\]
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 141) \[\underset{x\to a}{\mathop{\lim }}\,\frac{\sqrt{3x-a}-\sqrt{x+a}}{x-a}\]is equal to:
A)
\[\sqrt{2a}\]
done
clear
B)
\[\frac{1}{\sqrt{2a}}\]
done
clear
C)
2a
done
clear
D)
\[\frac{1}{2a}\]
done
clear
View Answer play_arrow
question_answer 142) \[\int_{0}^{1}{\frac{x}{{{(1-x)}^{3/4}}}dx}\]is equal to:
A)
\[\frac{-12}{5}\]
done
clear
B)
\[\frac{16}{5}\]
done
clear
C)
\[\frac{-16}{5}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 143) \[\int_{{}}^{{}}{\frac{{{x}^{5}}}{\sqrt{1+{{x}^{3}}}}}dx\]is equal to:
A)
\[\frac{2}{9}{{(1+{{x}^{3}})}^{3/2}}+c\]
done
clear
B)
\[\frac{2}{9}{{(1+{{x}^{3}})}^{3/2}}+\frac{2}{3}{{(11-{{x}^{3}})}^{1/2}}+c\]
done
clear
C)
\[\frac{2}{9}{{(1+{{x}^{3}})}^{3/2}}-\frac{2}{3}{{(1+{{x}^{3}})}^{1/2}}+c\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 144) \[\int_{1}^{2}{\log x\,dx}\]is equal to:
A)
\[\log \left( \frac{e}{2} \right)\]
done
clear
B)
\[\log \left( \frac{2}{e} \right)\]
done
clear
C)
\[\log \left( \frac{e}{4} \right)\]
done
clear
D)
\[\log \left( \frac{4}{e} \right)\]
done
clear
View Answer play_arrow
question_answer 145) The value of a for which the function \[f(x)=a\sin x+\frac{1}{3}\sin 3x\] has an extremum at \[x=\frac{\pi }{3},\] is:
A)
1
done
clear
B)
\[-1\]
done
clear
C)
0
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 146) If \[\theta \]is the angle between the vectors \[\vec{a}=2\hat{i}-\hat{j}+\hat{k}\]and \[\vec{b}=\hat{i}+2\hat{j}+\hat{k},\] then the angle is:
A)
1
done
clear
B)
\[{{\cos }^{-1}}\frac{1}{6}\]
done
clear
C)
\[\frac{1}{\sqrt{6}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 147) Two dice are thrown simultaneously. The probability that the sum of the points on two dice will be 7, is :
A)
5/36
done
clear
B)
1/6
done
clear
C)
7/36
done
clear
D)
2/9
done
clear
View Answer play_arrow
question_answer 148) Two parallel forces not having the same line of action form a couple, if they are:
A)
like and unlike
done
clear
B)
like and equal
done
clear
C)
unequal and unlike
done
clear
D)
equal and unlike
done
clear
View Answer play_arrow
question_answer 149) A particle is projected from the top of a tower \[h\] metres high and at the same moment particle is projected upwards from the bottom of the tower. If the two particles meet when the upper one has described \[(1/n)th\]of the distance, then the velocity of the projection of the lower particle is:
A)
\[\sqrt{n\,g\,h}\]
done
clear
B)
\[\frac{1}{2}\sqrt{n\,g\,h}\]
done
clear
C)
\[\sqrt{2\,n\,g\,h}\]
done
clear
D)
\[\sqrt{\frac{1}{2}n\,g\,h}\]
done
clear
View Answer play_arrow
question_answer 150) A particle is projected with a velocity \[{{v}_{0}}\]so that its range on a horizontal plane is twice the greatest height attained. The range is:
A)
\[\frac{5}{4g}v_{0}^{2}\]
done
clear
B)
\[\frac{4}{5g}v_{0}^{2}\]
done
clear
C)
\[\frac{4}{3}v_{0}^{2}\]
done
clear
D)
\[\frac{3}{5}v_{0}^{2}\]
done
clear
View Answer play_arrow
 \[\text{1}\text{.2V}\]
\[\text{1}\text{.2V}\] 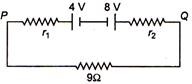 \[\frac{\text{1}}{\text{12}}\text{A}\,\,\text{and}\,\text{12}\,\text{V}\]
\[\frac{\text{1}}{\text{12}}\text{A}\,\,\text{and}\,\text{12}\,\text{V}\]  \[>\frac{a}{g}\]
\[>\frac{a}{g}\]