A) 4.9 \[\overset{o}{\mathop{A}}\,\]
B) 4.3 \[\overset{o}{\mathop{A}}\,\]
C) 3.8 \[\overset{o}{\mathop{A}}\,\]
D) 3.4 \[\overset{o}{\mathop{A}}\,\]
Correct Answer: B
Solution :
Key Idea: Diameter of an atom is defined as centre-to-centre distance between two atoms. The body, centered cubic structure of sodium is as shown: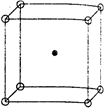 The distance (2r), where r is radius of an atom, can be defined as the centre-to-centre distance between two atoms packed as tightly, together as possible. This provides a type of effective radius for the atom and is sometime called the atomic radius. \[\therefore \] \[2\,r=3.7\] \[\Rightarrow \] \[r=\frac{3.7}{2}=1.85\,\overset{o}{\mathop{A}}\,\] The spacing between unit cells in various directions are called its lattice parameter a. Hence, in bcc lattice \[r=\frac{\sqrt{3}}{4}\,a\] (a is lattice para meter) \[1.85=\frac{\sqrt{3}}{4}\,a\] \[\Rightarrow \] \[a=\frac{1.85\times 4}{\sqrt{3}}=4.3\,\overset{o}{\mathop{A}}\,\]
The distance (2r), where r is radius of an atom, can be defined as the centre-to-centre distance between two atoms packed as tightly, together as possible. This provides a type of effective radius for the atom and is sometime called the atomic radius. \[\therefore \] \[2\,r=3.7\] \[\Rightarrow \] \[r=\frac{3.7}{2}=1.85\,\overset{o}{\mathop{A}}\,\] The spacing between unit cells in various directions are called its lattice parameter a. Hence, in bcc lattice \[r=\frac{\sqrt{3}}{4}\,a\] (a is lattice para meter) \[1.85=\frac{\sqrt{3}}{4}\,a\] \[\Rightarrow \] \[a=\frac{1.85\times 4}{\sqrt{3}}=4.3\,\overset{o}{\mathop{A}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
