A) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
B) \[[Ni{{(CO)}_{4}}]\]
C) \[{{[Ni{{(Cl)}_{4}}]}^{2-}}\]
D) All of the above
Correct Answer: A
Solution :
Key Idea: Draw the excited state and find hybridisation in order to find shape of molecule. \[Ni(CN)_{4}^{2-}\]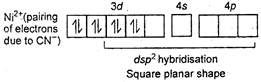 \[{{[NiC{{l}_{4}}]}^{2-}}\] Ni is in +2 oxidation state.
\[{{[NiC{{l}_{4}}]}^{2-}}\] Ni is in +2 oxidation state. 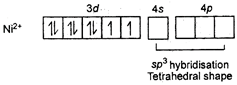 \[NiC{{(CO)}_{4}}\] Tetrahedral shape Oxidation state of Ni is zero
\[NiC{{(CO)}_{4}}\] Tetrahedral shape Oxidation state of Ni is zero 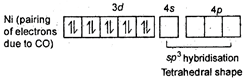
You need to login to perform this action.
You will be redirected in
3 sec
