A) 1 : 1
B) 2 : 1
C) 2 : 3
D) 3 : 2
Correct Answer: D
Solution :
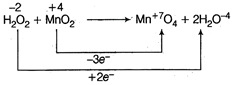 (Oxidation number two 0 atoms taken) On balancing of oxidation number \[3{{H}_{2}}{{O}_{2}}+2Mn{{O}_{2}}\xrightarrow{{}}2MnO_{4}^{-}+6{{H}_{2}}O\] Thus. \[{{H}_{2}}{{O}_{2}}:Mn{{O}_{2}}=3:2\]
(Oxidation number two 0 atoms taken) On balancing of oxidation number \[3{{H}_{2}}{{O}_{2}}+2Mn{{O}_{2}}\xrightarrow{{}}2MnO_{4}^{-}+6{{H}_{2}}O\] Thus. \[{{H}_{2}}{{O}_{2}}:Mn{{O}_{2}}=3:2\]
You need to login to perform this action.
You will be redirected in
3 sec
