A) \[CC{{l}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,HC{{H}_{2}}Cl\]
B) \[CC{{l}_{3}}\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,H\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\]
C) \[CC{{l}_{3}}\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}OH\]
D) \[CC{{l}_{3}}\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,{{H}_{2}}\]
Correct Answer: C
Solution :
Since, \[CC{{l}_{3}}\] is an electron withdrawing group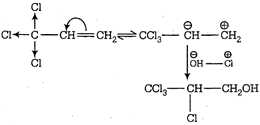 Thus, product P is \[CC{{l}_{3}}\,\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}OH\]
Thus, product P is \[CC{{l}_{3}}\,\underset{\begin{smallmatrix} | \\ Cl \end{smallmatrix}}{\mathop{C}}\,H-C{{H}_{2}}OH\]
You need to login to perform this action.
You will be redirected in
3 sec
