question_answer 1)
Consider the following statements: The total energy of a particle executing simple harmonic motion depends on its: (I) amplitude (II) period (III) displacement
Of these statements:
A)
I and II are correct
done
clear
B)
II and III are correct
done
clear
C)
I and III are correct
done
clear
D)
I, II and III are correct
done
clear
View Answer play_arrow
question_answer 2) The maximum velocity of a simple harmonic motion represented by \[y=3\sin \left( 100\,t+\frac{\pi }{6} \right)\,\,m\] is given by :
A)
300 m/s
done
clear
B)
\[\frac{3\pi }{6}\] m/s
done
clear
C)
100 m/s
done
clear
D)
\[\frac{\pi }{6}\] m/s
done
clear
View Answer play_arrow
question_answer 3) When earth moves round the sun, the quantity which remains constant is :
A)
angular velocity
done
clear
B)
kinetic energy
done
clear
C)
potential energy
done
clear
D)
area! Velocity
done
clear
View Answer play_arrow
question_answer 4) 1 kg body explodes into three fragments. The ratio of their masses is 1:1:3. The fragments of same mass move perpendicular to each other with speeds 30 m/s, while the heavier part remains in the initial direction. The speed of heavier part is :
A)
\[\frac{10}{\sqrt{2}}\] m/s
done
clear
B)
\[10\sqrt{2}\]m/s
done
clear
C)
\[20\sqrt{2}\]m/s
done
clear
D)
\[30\sqrt{2}\] m/s
done
clear
View Answer play_arrow
question_answer 5) The dimensional formula for the gravitational constant is :
A)
\[[{{M}^{-1}}{{L}^{3}}{{T}^{-2}}]\]
done
clear
B)
\[[ML{{T}^{-2}}]\]
done
clear
C)
\[[M{{L}^{2}}{{T}^{-2}}]\]
done
clear
D)
\[[{{M}^{-1}}{{L}^{-3}}T]\]
done
clear
View Answer play_arrow
question_answer 6) A particle is moving in a circle of radius R with constant speed v. If radius is doubled, then its centripetal force to keep the same speed gets:
A)
twice as great as before
done
clear
B)
half
done
clear
C)
one-fourth
done
clear
D)
remains constant
done
clear
View Answer play_arrow
question_answer 7) If linear density of a rod of length 3 m varies as\[\lambda =2+x\], then the position of the centre of gravity of the rod is :
A)
\[\frac{7}{3}\] m
done
clear
B)
\[\frac{12}{7}\]m
done
clear
C)
\[\frac{10}{7}\]m
done
clear
D)
\[\frac{9}{7}\]m
done
clear
View Answer play_arrow
question_answer 8)
A block of mass m initially at rest is dropped from a height h on to a spring of force constant k. The maximum compression in the spring is t, then:
A)
\[mgh=\frac{1}{2}k\,\,{{x}^{2}}\]
done
clear
B)
\[mg\,(h+x)=\frac{1}{2}k\,\,{{x}^{2}}\]
done
clear
C)
\[mgh=\frac{1}{2}\,k\,\,{{x}^{2}}\]
done
clear
D)
\[mg\,(h+x)=\frac{1}{2}\,k\,{{(x+h)}^{2}}\]
done
clear
View Answer play_arrow
question_answer 9) A ball of mass m moves with speed v and it strikes normally with a wall and reflected back normally. If its time of contact with wall is t, then find force exerted by ball on the wall.
A)
\[\frac{2\,mv}{t}\]
done
clear
B)
\[\frac{mv}{t}\]
done
clear
C)
\[mvt\]
done
clear
D)
\[\frac{mv}{2t}\]
done
clear
View Answer play_arrow
question_answer 10) A wheel of radius 1m rolls forward half a revolution on a horizontal ground. The magnitude of the displacement of the point of the wheel initially in contact with the ground is:
A)
\[2\,\,\pi \]
done
clear
B)
\[\sqrt{2\,\,\pi }\]
done
clear
C)
\[\sqrt{{{\pi }^{2}}+4}\]
done
clear
D)
\[\pi \]
done
clear
View Answer play_arrow
question_answer 11) Water is flowing in a pipe of diameter 4 cm with a velocity 3 m/s. The water then enters into a pipe of diameter 2 cm. The velocity of water in the other pipe is :
A)
3 m/s
done
clear
B)
6 m/s
done
clear
C)
12 m/s
done
clear
D)
8 m/s
done
clear
View Answer play_arrow
question_answer 12) The surface tension of a liquid is 5 N/m. If a film is held on a ring of area 0.02 \[{{m}^{2}}\], its total surface energy is about:
A)
\[2\times {{10}^{-2}}J\]
done
clear
B)
\[2.5\times {{10}^{-2}}J\]
done
clear
C)
\[2\times {{10}^{-1}}J\]
done
clear
D)
\[3\times {{10}^{-1}}J\]
done
clear
View Answer play_arrow
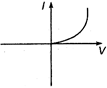
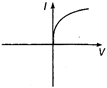
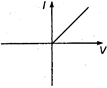
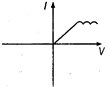
question_answer 13) A viscous fluid is flowing through a cylindrical tube. The velocity distribution of the fluid is best represented by the diagram :
A)
done
clear
B)
done
clear
C)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 14) A wire is stretched by 1 mm by a force of 1 kN. The work done in stretching the wire is :
A)
5 erg
done
clear
B)
5 J
done
clear
C)
0.5 erg
done
clear
D)
0.5 J
done
clear
View Answer play_arrow
question_answer 15) In isochoric process :
A)
\[\Delta Q=\Delta Q\]
done
clear
B)
\[\Delta Q=\Delta W\]
done
clear
C)
\[\Delta U=\Delta W\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 16) Which is not a path function?
A)
\[\Delta Q\]
done
clear
B)
\[\Delta Q+\Delta W\]
done
clear
C)
\[\Delta W\]
done
clear
D)
\[\Delta Q-\Delta W\]
done
clear
View Answer play_arrow
question_answer 17) The work done, W during an isothermal process in which 1 mole of the gas expands from an initial volume \[{{V}_{1}}\] to a final volume \[{{V}_{2}}\]is given by : T = gas constant, T = temperature)
A)
\[R\,({{V}_{2}}-{{V}_{1}}){{\log }_{e}}\left( \frac{{{T}_{1}}}{{{T}_{2}}} \right)\]
done
clear
B)
\[R\,({{T}_{2}}-{{T}_{1}}){{\log }_{e}}\left( \frac{{{V}_{1}}}{{{V}_{2}}} \right)\]
done
clear
C)
\[RT\,{{\log }_{e}}\left( \frac{{{V}_{2}}}{{{V}_{1}}} \right)\]
done
clear
D)
\[2RT\,{{\log }_{e}}\left( \frac{{{V}_{1}}}{{{V}_{2}}} \right)\]
done
clear
View Answer play_arrow
question_answer 18) The P-V diagram of a system undergoing thermodynamic transformation is shown in figure. The work done by the system in going from \[A\to B\to C\] is 30 J, and 40 J heat is given to the system. The change in internal energy between A and C is :
A)
10 J
done
clear
B)
70 J
done
clear
C)
84 J
done
clear
D)
134 J
done
clear
View Answer play_arrow
question_answer 19) The degrees of freedom of a molecule of a triatomic gas are :
A)
2
done
clear
B)
4
done
clear
C)
6
done
clear
D)
8
done
clear
View Answer play_arrow
question_answer 20) Ozone layer blocks the radiations of wave- length:
A)
less than \[3\times {{10}^{-7}}m\]
done
clear
B)
equal to \[3\times {{10}^{-7}}m\]
done
clear
C)
more than \[3\times {{10}^{-7}}m\]
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 21) Two simple harmonic waves of the same amplitude and frequency differ by a phase\[\pi /2\]. When they are fed simultaneously to the X and y-plates of a CRO, the screen would display the trace of:
A)
a circle
done
clear
B)
an ellipse
done
clear
C)
a straight line
done
clear
D)
a square
done
clear
View Answer play_arrow
question_answer 22) The speed of a wave is 360 m/s and frequency is 500 Hz. Phase difference between two consecutive particles is \[{{60}^{o}}\], then path difference between them will be :
A)
0.72 cm
done
clear
B)
120 cm
done
clear
C)
12 cm
done
clear
D)
7.2 cm
done
clear
View Answer play_arrow
question_answer 23) A string of length 2 m is fixed at both ends. If this string vibrates in its fourth normal mode with a frequency of 500 Hz, then the waves would travel on it with a velocity of:
A)
125 m/s
done
clear
B)
250 m/s
done
clear
C)
500 m/s
done
clear
D)
1000 m/s
done
clear
View Answer play_arrow
question_answer 24) The fundamental frequency of a sonometer wire is n. If its radius is doubled and its tension becomes half, the material of the wire remains same, the new fundamental frequency will be:
A)
n
done
clear
B)
\[\frac{n}{\sqrt{2}}\]
done
clear
C)
\[\frac{n}{2}\]
done
clear
D)
\[\frac{n}{2\sqrt{2}}\]
done
clear
View Answer play_arrow
question_answer 25) In open organ pipe, if fundamental frequency is n, then the other frequencies are :
A)
n, 2 n, 3n, 4n
done
clear
B)
n, 3n, 5n
done
clear
C)
n, 2n, 4n, 8n
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 26) A sound wave of frequency \[f\] propagating through air with a velocity c, is reflected from a surface which is moving away from the source with a constant speed v. The frequency of the reflected wave, measured by the observer the position of the source is:
A)
\[\frac{f(c-v)}{c+v}\]
done
clear
B)
\[\frac{f(c+v)}{c-v}\]
done
clear
C)
\[\frac{f(c+2v)}{c+v}\]
done
clear
D)
\[\frac{f(c-v)}{c-2v}\]
done
clear
View Answer play_arrow
question_answer 27) A convex lens of focal length 10 cm and image formed by it, is at least distance of distinct vision then the magnifying power is:
A)
3.5
done
clear
B)
2.5
done
clear
C)
1.5
done
clear
D)
1.4
done
clear
View Answer play_arrow
question_answer 28) If two +5 D. lenses are mounted at some distance apart, the equivalent power will always be negative, if the distance is
A)
greater than 40 cm
done
clear
B)
equal to 40 cm
done
clear
C)
equal to 10 cm
done
clear
D)
less than 10 cm
done
clear
View Answer play_arrow
question_answer 29) A Galilean telescope has an objective of focal length 100 cm and magnifying power 50. The distance between the two lenses in normal adjustment will be
A)
98 cm
done
clear
B)
100 cm
done
clear
C)
150 cm
done
clear
D)
200 cm
done
clear
View Answer play_arrow
question_answer 30) A film projector magnifies a 100 \[c{{m}^{2}}\] film strip on a screen. If the linear magnification is 4, the area of the magnified film on the screen is:
A)
1600 \[c{{m}^{2}}\]
done
clear
B)
400 \[c{{m}^{2}}\]
done
clear
C)
800 \[c{{m}^{2}}\]
done
clear
D)
200 \[c{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 31) The exposure time of a camera lens at \[f/2.8\] setting is 1/200 s. The correct exposure time at \[f/5.6\] setting is:]
A)
0.02 s
done
clear
B)
0.04 s
done
clear
C)
0.20 s
done
clear
D)
0.40s
done
clear
View Answer play_arrow
question_answer 32) In Youngs double slit experiment, the slit width and the distance of slits from the screen both are doubled. The fringe width:
A)
increases
done
clear
B)
decreases
done
clear
C)
remains unchanged
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 33) In Youngs double slit experiment, the aperture screen distance is 2 m. The slit width is 1 mm. Light of 600 nm is used. If a thin plate of glass\[(\mu =1.5)\] of thickness 0.06 mm is placed over one of the slits, then there will be a lateral displacement of the fringes by :
A)
zero
done
clear
B)
6 cm
done
clear
C)
10 cm
done
clear
D)
15 cm
done
clear
View Answer play_arrow
question_answer 34) A single slit is used to observe diffraction pattern with red light. On replacing the red light with violet light the diffraction pattern would:
A)
remain unchanged
done
clear
B)
become narrower
done
clear
C)
become broader
done
clear
D)
disappear
done
clear
View Answer play_arrow
question_answer 35) The resistance of a discharge tube is-
A)
zero
done
clear
B)
ohmic
done
clear
C)
non-ohmic
done
clear
D)
infinity
done
clear
View Answer play_arrow
question_answer 36) Which one of the following processes depends on gravity?
A)
Conduction
done
clear
B)
Convection
done
clear
C)
Radiation
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 37) The radius of solid metallic non-conducting sphere is 60 cm and charge on the sphere is 500\[\mu C\]. The electric field at a distance 10 cm from centre of sphere is :
A)
\[2\times {{10}^{6}}N/C\]
done
clear
B)
\[2\times {{10}^{8}}N/C\]
done
clear
C)
\[5\times {{10}^{6}}N/C\]
done
clear
D)
\[5\times {{10}^{8}}N/C\]
done
clear
View Answer play_arrow
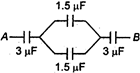
question_answer 38)
The equivalent capacitance between the points A and B in the following circuit is :
A)
\[1\,\mu F\]
done
clear
B)
\[2\,\mu F\]
done
clear
C)
\[4\,\mu F\]
done
clear
D)
\[8\,\mu F\]
done
clear
View Answer play_arrow
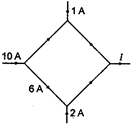
question_answer 39)
The figure shows a network of currents. The magnitude of current is shown here The current/will be:
A)
3 A
done
clear
B)
9 A
done
clear
C)
13 A
done
clear
D)
19 A
done
clear
View Answer play_arrow
question_answer 40)
If resistance of voltmeter is 10000 \[\Omega \] and resistance of galvanometer is\[2\,\Omega \], then find R when voltmeter reads 12 V and galvanometer reads 0.1 A.
A)
118 \[\Omega \]
done
clear
B)
120 \[\Omega \]
done
clear
C)
124 \[\Omega \]
done
clear
D)
114 \[\Omega \]
done
clear
View Answer play_arrow
question_answer 41) Two bulbs 25 W; 220 V and 100 W, 220 V are given. Which has higher resistance?
A)
25 W bulb
done
clear
B)
100 W bulb
done
clear
C)
Both bulbs will have equal resistance
done
clear
D)
Resistance of bulbs cannot be compared
done
clear
View Answer play_arrow
question_answer 42) An electron (mass \[=9.1\times {{10}^{-31}}kg\], charge \[1.16\times {{10}^{-19}}C\]) experiences no deflection, if subjected to an electric field of \[3.32\times {{10}^{5}}\] V/m, and a magnetic field of \[2.0\times {{10}^{-3}}Wb/{{m}^{2}}\] . Both the fields are normal to the path of electron and to each other. If the electric field is removed, then the electron will revolve in an orbit of radius :
A)
45 m
done
clear
B)
4.5 m
done
clear
C)
0.45 m
done
clear
D)
0.045 m
done
clear
View Answer play_arrow
question_answer 43) An LCR circuit of \[R=100\,\Omega \] is connected to an AC source 100 V, 50 Hz. The magnitude of phase difference between current and voltage is \[{{30}^{o}}\]. The power dissipated in the LCR circuit is:
A)
50 W
done
clear
B)
86.6 W
done
clear
C)
100 W
done
clear
D)
200 W
done
clear
View Answer play_arrow
question_answer 44) If half-life of radium is 77 days, its decay constant will be :
A)
\[3\times {{10}^{-3}}\]/day
done
clear
B)
\[9\times {{10}^{-3}}\]/day
done
clear
C)
\[1\times {{10}^{-3}}\]/day
done
clear
D)
\[6\times {{10}^{-3}}\]/day
done
clear
View Answer play_arrow
question_answer 45) In a radioactive reaction \[_{92}{{X}^{232}}{{\to }_{82}}{{X}^{204}}\] the number of a-particles emitted is :
A)
7
done
clear
B)
6
done
clear
C)
5
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 46) According to Bubbles law, the red-shift (Z) of a receding galaxy and its distance r from earth are related as:
A)
\[Z\propto r\]
done
clear
B)
\[Z\propto \frac{1}{r}\]
done
clear
C)
\[Z\propto \frac{1}{{{r}^{2}}}\]
done
clear
D)
\[Z\propto {{r}^{3/2}}\]
done
clear
View Answer play_arrow
question_answer 47) The de-Broglie wavelength of a body of mass and kinetic energy E is given by:_
A)
\[\lambda =\frac{h}{mE}\]
done
clear
B)
\[\lambda =\frac{\sqrt{2\,mE}}{h}\]
done
clear
C)
\[\lambda =\frac{h}{2\,mE}\]
done
clear
D)
\[\lambda =\frac{h}{\sqrt{2\,mE}}\]
done
clear
View Answer play_arrow
question_answer 48) Different voltages are applied across a p-n junction and the currents are measured from each value. Which of the following graphs is obtained between voltage and current?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 49)
A logic gate having two inputs A and B and output C has the following truth table
A
B
C
1
1
0
1
0
1
0
1
1
0
0
1
It is :
A)
an OR gate
done
clear
B)
an AND gate
done
clear
C)
a NOR gate
done
clear
D)
a NAND gate
done
clear
View Answer play_arrow
question_answer 50) For sky wave propagation of 10 MHz signal, what should be the minimum electron density in ionosphere?
A)
\[\sim 1.2\times {{10}^{12}}{{m}^{-3}}\]
done
clear
B)
\[\sim {{10}^{6}}{{m}^{-3}}\]
done
clear
C)
\[\sim {{10}^{4}}{{m}^{-3}}\]
done
clear
D)
\[\sim {{10}^{22}}{{m}^{-3}}\]
done
clear
View Answer play_arrow
question_answer 51) The number of molecules of \[C{{O}_{2}}\] present in 44 g of \[C{{O}_{2}}\] is :
A)
\[6.0\times {{10}^{23}}\]
done
clear
B)
\[3\times {{10}^{23}}\]
done
clear
C)
\[12\times {{10}^{23}}\]
done
clear
D)
\[3\times {{10}^{10}}\]
done
clear
View Answer play_arrow
question_answer 52) Magnitude of kinetic energy in an orbit is equal to :
A)
half of the potential energy
done
clear
B)
twice of the potential energy
done
clear
C)
one fourth of the potential energy
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 53) A p-orbital in a given shell can accommodate upto:
A)
four electrons
done
clear
B)
two electrons with parallel spin
done
clear
C)
six electrons
done
clear
D)
two electrons with opposite spin
done
clear
View Answer play_arrow
question_answer 54) Which of the following is Heisenberg uncertainty principle?
A)
\[\Delta x\,.\,\Delta p\ge \frac{h}{4\pi }\]
done
clear
B)
\[\Delta x\,.\,\Delta p=\frac{h}{4\pi }\]
done
clear
C)
\[\Delta x\,.\,\Delta p\le \frac{h}{4\pi }\]
done
clear
D)
\[\Delta x\,.\,\Delta p<\frac{h}{4\pi }\]
done
clear
View Answer play_arrow
question_answer 55) Chromium is represented by the electronic configuration :
A)
\[[Ne]\,3{{s}^{2}}3{{p}^{6}}3{{d}^{1}}4{{s}^{2}}\]
done
clear
B)
\[[Ne]\,3{{s}^{2}}3{{p}^{6}}3{{d}^{2}}4{{s}^{1}}\]
done
clear
C)
\[[Ne]\,3{{s}^{2}}3{{p}^{6}}3{{d}^{5}}4{{s}^{1}}\]
done
clear
D)
\[[Ne]\,3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{4}}\]
done
clear
View Answer play_arrow
question_answer 56) Number of neutron in \[{{C}^{12}}\]is :
A)
61
done
clear
B)
7
done
clear
C)
8
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 57) H-bond is not present in :
A)
water
done
clear
B)
glycerol
done
clear
C)
hydrogen fluoride
done
clear
D)
hydrogen sulphide
done
clear
View Answer play_arrow
question_answer 58) Oxidation number of S in \[SO_{4}^{2-}\]:
A)
+6
done
clear
B)
+3
done
clear
C)
+2
done
clear
D)
-2
done
clear
View Answer play_arrow
question_answer 59) 2g of \[{{O}_{2}}\] at \[{{270}^{o}}C\] and 760 mm of Hg pressure has volume :
A)
1.4 L
done
clear
B)
2.8 L
done
clear
C)
11.2L
done
clear
D)
22.4 L
done
clear
View Answer play_arrow
question_answer 60) If pressure increases then its effect on given equilibrium \[2NO\,(g){{N}_{2}}(g)+{{O}_{2}}(g)\] is shift in:
A)
forward direction
done
clear
B)
backward direction
done
clear
C)
no effect
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 61) Which is Lewis base: \[{{I}_{2}}+{{I}^{-}}\xrightarrow{{}}{{I}_{3}}^{-}?\]
A)
\[{{I}_{2}}\]
done
clear
B)
\[{{I}_{3}}^{-}\]
done
clear
C)
\[{{I}^{-}}\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 62) The heat of neutralisation of any strong acid and a strong base is nearly equal to:
A)
-75.3kJ
done
clear
B)
+57.3kJ
done
clear
C)
-57.3kJ
done
clear
D)
+ 75.3kJ
done
clear
View Answer play_arrow
question_answer 63) pH of \[{{10}^{-8}}M\,HCl\] solution is:
A)
8
done
clear
B)
between 7 and 8
done
clear
C)
between 6 and 7
done
clear
D)
between 8 and 9
done
clear
View Answer play_arrow
question_answer 64) A reaction \[A\xrightarrow{{}}B\] follows a second order kinetic. Doubling the concentration of A will increase the rate of formation of B by a factor of:
A)
1/4
done
clear
B)
4
done
clear
C)
1/2
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 65) Which of the following is always negative for exothermic reaction?
A)
\[\Delta H\]
done
clear
B)
\[\Delta S\]
done
clear
C)
\[\Delta G\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 66) Unit of equivalent conductance is :
A)
\[oh{{m}^{-1}}c{{m}^{2}}\,{{(g-eq)}^{-1}}\]
done
clear
B)
\[ohm\,\,cm\,(g-eq)\]
done
clear
C)
\[ohm\,\,c{{m}^{2}}\,{{(g-eq)}^{-1}}\]
done
clear
D)
\[oh{{m}^{-1}}\,cm\,{{(g-eq)}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 67) For cell reaction \[Zn+C{{u}^{2+}}\xrightarrow{{}}Z{{n}^{2+}}+Cu\] cell representation is:
A)
\[C{{u}^{2+}}|Zn||Z{{n}^{2+}}|Cu\]
done
clear
B)
\[Cu|C{{u}^{2+}}||Z{{n}^{2+}}|Zn\]
done
clear
C)
\[Cu|Z{{n}^{2+}}||Zn|C{{u}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}|Zn||Z{{n}^{2+}}|Cu\]
done
clear
View Answer play_arrow
question_answer 68) Molal solution means 1 mole of solute present in:
A)
1000 g of solvent
done
clear
B)
1 L of solvent
done
clear
C)
1 Lot solution
done
clear
D)
1000g of solution
done
clear
View Answer play_arrow
question_answer 69) Which of the following shows maximum depression in freezing point?
A)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
B)
\[NaCl\]
done
clear
C)
Urea
done
clear
D)
Glucose
done
clear
View Answer play_arrow
question_answer 70) An emulsion is a colloidal dispersion of:
A)
a liquid in a gas
done
clear
B)
a liquid in a liquid
done
clear
C)
a solid in a liquid
done
clear
D)
a gas in a solid
done
clear
View Answer play_arrow
question_answer 71) Size of colloidal particle is :
A)
1 nm
done
clear
B)
1-100 nm
done
clear
C)
< 100 nm
done
clear
D)
> 1000 nm
done
clear
View Answer play_arrow
question_answer 72) Ether show isomerism with:
A)
alcohol
done
clear
B)
ethane
done
clear
C)
halide
done
clear
D)
aldehyde
done
clear
View Answer play_arrow
question_answer 73) Which of the following shows geometrical isomerism?
A)
\[{{C}_{2}}{{H}_{5}}Br\]
done
clear
B)
\[(C{{H}_{2}})\,{{(COOH)}_{2}}\]
done
clear
C)
\[(C{{H}_{2}})\,{{(COOH)}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 74) Which of the following is present in natural gas?
A)
n-butane
done
clear
B)
Ethane
done
clear
C)
Methane
done
clear
D)
Propane
done
clear
View Answer play_arrow
question_answer 75) Which of the following have delocalized electron?
A)
Benzene
done
clear
B)
Cyclohexane
done
clear
C)
\[C{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
View Answer play_arrow
question_answer 76) Nitration of benzene is :
A)
electrophilic substitution
done
clear
B)
electrophilic addition
done
clear
C)
nucleophilic substitution
done
clear
D)
nucleophilic addition
done
clear
View Answer play_arrow
question_answer 77) Alkyl halide can be converted into alkene by :
A)
nucleophilic substitution reaction
done
clear
B)
elimination reaction
done
clear
C)
both nucleophilic substitution and elimination reaction
done
clear
D)
rearrangement
done
clear
View Answer play_arrow
question_answer 78) Which of the following compounds is most acidic?
A)
\[C{{H}_{4}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[CH\equiv CH\]
done
clear
D)
\[{{C}_{2}}{{H}_{5}}OH\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following reacts with \[KMn{{O}_{4}}\] but does not react with \[AgN{{O}_{3}}\]?
A)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
B)
\[C{{H}_{4}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 80) Which of the following compound does not show anti-Markownikoffs addition?
A)
Propene
done
clear
B)
1-butene
done
clear
C)
2-butene
done
clear
D)
2-pentene
done
clear
View Answer play_arrow
question_answer 81) Methyl ketone is identified by :
A)
lodoform test
done
clear
B)
Fehling solution
done
clear
C)
Toilers reagent
done
clear
D)
Schiffs reagent
done
clear
View Answer play_arrow
question_answer 82) Which of the following does not give Fehling solution test?
A)
Acetone
done
clear
B)
Propanal
done
clear
C)
Ethanal
done
clear
D)
Butanal
done
clear
View Answer play_arrow
question_answer 83) Cyanide group on hydrolysis gives :
A)
acid
done
clear
B)
acetamide
done
clear
C)
amine
done
clear
D)
hydrate
done
clear
View Answer play_arrow
question_answer 84) In alkyl cyanide alkyl group attached with :
A)
C of CN group
done
clear
B)
N of CN group
done
clear
C)
either C or N of CN group
done
clear
D)
both C and N of CN group
done
clear
View Answer play_arrow
question_answer 85) Fat on hydrolysis gives which alcohol?
A)
Glycerol
done
clear
B)
Propanol
done
clear
C)
Butanol
done
clear
D)
Ethanol
done
clear
View Answer play_arrow
question_answer 86) Which of the following is natural polymer?
A)
PVC
done
clear
B)
Nylon 66
done
clear
C)
Teflon
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 87) Condensation product of caprolactum is :
A)
Nylon 6
done
clear
B)
Nylon 66
done
clear
C)
Nylon 60
done
clear
D)
Nylon 6,10
done
clear
View Answer play_arrow
question_answer 88) Which inert gas have highest foiling point?
A)
Xe
done
clear
B)
Ar
done
clear
C)
Kr
done
clear
D)
He
done
clear
View Answer play_arrow
question_answer 89) In Ostwald process of manufacturing of \[HN{{O}_{3}}\], catalyst used is:
A)
Mo
done
clear
B)
Fe
done
clear
C)
Mn
done
clear
D)
Pt
done
clear
View Answer play_arrow
question_answer 90) Important ore of Mg is :
A)
Gypsum
done
clear
B)
Carnallite
done
clear
C)
Magnatide
done
clear
D)
Carnolite
done
clear
View Answer play_arrow
question_answer 91) Purification of Al by electrolysis method is called:
A)
Halls process
done
clear
B)
Baeyer process
done
clear
C)
Ostwald process
done
clear
D)
Hoopes process
done
clear
View Answer play_arrow
question_answer 92) Decreasing size of ions is:
A)
\[I>{{I}^{-}}>{{I}^{+}}\]
done
clear
B)
\[{{I}^{-}}>I>{{I}^{+}}\]
done
clear
C)
\[{{I}^{+}}>{{I}^{-}}>I\]
done
clear
D)
\[I>{{I}^{+}}>{{I}^{-}}\]
done
clear
View Answer play_arrow
question_answer 93) Which of the following is kept in water?
A)
White phosphorus
done
clear
B)
Sodium
done
clear
C)
Potassium
done
clear
D)
Calcium
done
clear
View Answer play_arrow
question_answer 94) When heated \[N{{H}_{3}}\] is passed over \[CuO\] gas evolved is :
A)
\[{{N}_{2}}\]
done
clear
B)
\[{{N}_{2}}O\]
done
clear
C)
\[HN{{O}_{3}}\]
done
clear
D)
\[N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 95) Formula of hypophosphorus acid is :
A)
\[H-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{P}}\,}}\,-OH\]
done
clear
B)
\[HO-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{P}}\,}}\,-OH\]
done
clear
C)
\[H-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\mathop{P}}\,}}\,=OH\]
done
clear
D)
\[H-\overset{\begin{smallmatrix} O \\ || \end{smallmatrix}}{\mathop{\underset{\begin{smallmatrix} | \\ OH \end{smallmatrix}}{\mathop{P}}\,}}\,-OH\]
done
clear
View Answer play_arrow
question_answer 96) Most powerful reducing agent is :
A)
Li
done
clear
B)
Na
done
clear
C)
Ca
done
clear
D)
Mg
done
clear
View Answer play_arrow
question_answer 97) When calomel reacts with \[N{{H}_{4}}OH\] solution the compound formed is :
A)
\[N{{H}_{2}}=Hg-Cl\]
done
clear
B)
\[H{{g}_{2}}C{{l}_{2}}N{{H}_{3}}\]
done
clear
C)
\[Hg{{(N{{H}_{3}})}_{2}}C{{l}_{2}}\]
done
clear
D)
\[HgC{{l}_{2}}N{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 98) Which of the following is coloured compound?
A)
\[Cu{{F}_{2}}\]
done
clear
B)
\[Cul\]
done
clear
C)
\[NaCl\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 99) \[{{H}_{2}}{{O}_{2}}\] is formed by which of the following compounds?
A)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
B)
\[NaOH\]
done
clear
C)
\[N{{a}_{2}}O\]
done
clear
D)
\[K{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 100) Copper sulphate solution reacts with KCN and gives:
A)
\[{{K}_{3}}[Cu{{(CN)}_{4}}]\]
done
clear
B)
\[CuCN\]
done
clear
C)
\[Cu{{(CN)}_{2}}\]
done
clear
D)
\[{{K}_{2}}[Cu{{(CN)}_{4}}]\]
done
clear
View Answer play_arrow
question_answer 101) Which one is absent in free-state during origin of life?
A)
\[{{O}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]
done
clear
C)
\[{{N}_{3}}\]
done
clear
D)
\[N{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 102) Peripatus is connecting link between:
A)
Arthropoda and Arinelida
done
clear
B)
Arthropoda and Mollusca
done
clear
C)
Arthropoda and Echinodermata
done
clear
D)
Arthropoda and Platyhelminthes
done
clear
View Answer play_arrow
question_answer 103) Theory of natural selection is given by:
A)
Charles Darwin
done
clear
B)
Wallace
done
clear
C)
J.B. Lamarck
done
clear
D)
Mendel
done
clear
View Answer play_arrow
question_answer 104) Modern synthetic theory of evolution not involved:
A)
gene mutation
done
clear
B)
gene recombination
done
clear
C)
natural selection
done
clear
D)
increase in size
done
clear
View Answer play_arrow
question_answer 105) Number of peristomial teeth in moss is :
A)
16 + 16
done
clear
B)
16 + 32
done
clear
C)
8 + 16
done
clear
D)
32 + 32
done
clear
View Answer play_arrow
question_answer 106) Reserve food material of algae is :
A)
starch
done
clear
B)
glycogen
done
clear
C)
fat
done
clear
D)
sugar
done
clear
View Answer play_arrow
question_answer 107) Multinucleated filament of Rhizopus is :
A)
coenocytic
done
clear
B)
conidia
done
clear
C)
heterothallus
done
clear
D)
homothallus
done
clear
View Answer play_arrow
question_answer 108) Classification based on chromosome number is:
A)
cytotaxonomy
done
clear
B)
numerical taxonomy
done
clear
C)
karyotaxonomy
done
clear
D)
biochemistry
done
clear
View Answer play_arrow
question_answer 109) Noh-chordates have :
A)
notochord
done
clear
B)
dorsal tubular nerve chord
done
clear
C)
pharyngeal gill cleft
done
clear
D)
absence of hepatic portal system
done
clear
View Answer play_arrow
question_answer 110) Sea anemone belong to phylum :
A)
Protozoa
done
clear
B)
Porifera
done
clear
C)
Coelenterata
done
clear
D)
Echinodermata
done
clear
View Answer play_arrow
question_answer 111) The males of honey-bee are produced by:
A)
sexually
done
clear
B)
budding
done
clear
C)
spore formation
done
clear
D)
parthenogenesis
done
clear
View Answer play_arrow
question_answer 112) Which of the following is maximum in chloroplast?
A)
RuBP carboxylase
done
clear
B)
Hexokinase
done
clear
C)
Phosphatase
done
clear
D)
Nuclease
done
clear
View Answer play_arrow
question_answer 113) Molecular weight of protein is :
A)
> 12000
done
clear
B)
< 6000
done
clear
C)
< 12000
done
clear
D)
600-3000
done
clear
View Answer play_arrow
question_answer 114) Which of the following is non-reducing sugar?
A)
Starch
done
clear
B)
Sucrose
done
clear
C)
Maltose
done
clear
D)
Galactose
done
clear
View Answer play_arrow
question_answer 115) Fat soluble vitamin is :
A)
A
done
clear
B)
B
done
clear
C)
C
done
clear
D)
Cholesterol
done
clear
View Answer play_arrow
question_answer 116) Richest energy compound is :
A)
creatinine phosphate
done
clear
B)
ATP
done
clear
C)
carbohydrate
done
clear
D)
fat
done
clear
View Answer play_arrow
question_answer 117) Which is the hereditary material in bacteria?
A)
Nucleic acid
done
clear
B)
Nucleic acid and cytoplasm
done
clear
C)
Nucleic acid and histone
done
clear
D)
None of the above
done
clear
View Answer play_arrow
question_answer 118) If a dwarf plant is treated with gibberellins it becomes tall and this plant now crosses with pure tall plant then progeny of first generation (Fi) is :
A)
all dwarf
done
clear
B)
all tall
done
clear
C)
75% tall and 25% dwarf
done
clear
D)
75 % dwarf and 25 % tall
done
clear
View Answer play_arrow
question_answer 119) Unit of nucleic acid is :
A)
nucleotide
done
clear
B)
nucleoside
done
clear
C)
nucleic acid
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 120) DNA replication requires :
A)
DNA polymerase only
done
clear
B)
DNA polymerase and ligase
done
clear
C)
Ligase only
done
clear
D)
RNA polymerase
done
clear
View Answer play_arrow
question_answer 121) Which of the following is not correct about translation?
A)
It starts with AUG
done
clear
B)
Stopped at termination codon
done
clear
C)
Based on operon model
done
clear
D)
Occurs in nucleus
done
clear
View Answer play_arrow
question_answer 122) Method by which information reaches from DNA to RNA is:
A)
transcription
done
clear
B)
translation
done
clear
C)
transformation
done
clear
D)
transduction
done
clear
View Answer play_arrow
question_answer 123) ABO blood group system is given by :
A)
Landsteiner
done
clear
B)
Wallace
done
clear
C)
de Vries
done
clear
D)
Lamarck
done
clear
View Answer play_arrow
question_answer 124) Edible part of tomato is :
A)
epiparp
done
clear
B)
pericap and placenta
done
clear
C)
mesocarp
done
clear
D)
thalamus
done
clear
View Answer play_arrow
question_answer 125) Which of the following is not true for double fertilization?
A)
Discovered by Nawaschin
done
clear
B)
Male gamete and secondary nucleus fused to form endosperm nucleus
done
clear
C)
Endosperm nucleus is diploid
done
clear
D)
Endosperm provides nutrition to embryo
done
clear
View Answer play_arrow
question_answer 126)
The floral formula,
A)
Allium cepa
done
clear
B)
Sunflower
done
clear
C)
Cucurbita
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 127) Vessels and companion cells are characteristics of:
A)
angiosperm
done
clear
B)
gymnosperm
done
clear
C)
pteridophyta
done
clear
D)
fern
done
clear
View Answer play_arrow
question_answer 128) Conjoint, collateral and closed vascular bundle is found in:
A)
monocot stem
done
clear
B)
monocot root
done
clear
C)
dicot stem
done
clear
D)
dicot root
done
clear
View Answer play_arrow
question_answer 129) Conglobate gland is present in :
A)
female cockroach
done
clear
B)
earthworm
done
clear
C)
male cockroach
done
clear
D)
honey bee
done
clear
View Answer play_arrow
question_answer 130) Function of clitellum is :
A)
formation of cocoon
done
clear
B)
excretion
done
clear
C)
respiration
done
clear
D)
circulation
done
clear
View Answer play_arrow
question_answer 131) The stage upto which glycolysis and fermentation is common is :
A)
dihydroxy acetone
done
clear
B)
3-phosphoglyceraldehyde
done
clear
C)
pyruvate
done
clear
D)
glucose-6-phosphate
done
clear
View Answer play_arrow
question_answer 132) Which of the following is not an intermediate of Krebs cycle?
A)
Acetyl Co-A
done
clear
B)
Citric acid
done
clear
C)
Succinic acid
done
clear
D)
Lactic acid
done
clear
View Answer play_arrow
question_answer 133) Dental formula of adult man is :
A)
\[\frac{2123}{2123}\]
done
clear
B)
\[\frac{2122}{2122}\]
done
clear
C)
\[\frac{2122}{2122}\]
done
clear
D)
\[\frac{2124}{2124}\]
done
clear
View Answer play_arrow
question_answer 134) Rennin secreted in which part of alimentary canal?
A)
Stomach
done
clear
B)
Kidney
done
clear
C)
Duodenum
done
clear
D)
Small intestine
done
clear
View Answer play_arrow
question_answer 135) \[C{{O}_{2}}\] transported mainly by :
A)
plasma
done
clear
B)
carbonic acid
done
clear
C)
bicarbonate
done
clear
D)
carboxyhaemoglobin
done
clear
View Answer play_arrow
question_answer 136) Respiratory quotient of carbohydrate is :
A)
unity
done
clear
B)
greater than unity
done
clear
C)
less than unity
done
clear
D)
equal to five
done
clear
View Answer play_arrow
question_answer 137) By which mechanism, oxygen is transported from lungs to cells?
A)
Diffusion
done
clear
B)
Facilitated diffusion
done
clear
C)
Transpiration
done
clear
D)
Osmosis
done
clear
View Answer play_arrow
question_answer 138) If ADH level of blood is less :
A)
volume of urine increases
done
clear
B)
volume of urine decreases
done
clear
C)
volume of urine is normal
done
clear
D)
volume of urine is unaffected
done
clear
View Answer play_arrow
question_answer 139) Enzyme present in sperm acrosome to dissolve egg membrane is :
A)
sperm lysine
done
clear
B)
ovolysin
done
clear
C)
spermatogenolysin
done
clear
D)
spermatocymin
done
clear
View Answer play_arrow
question_answer 140) Correct sequence in embryonic development of frog is :
A)
Zygote \[\to \] Cleavage \[\to \] Blastula \[\to \] Gastrula
done
clear
B)
Zygote \[\to \] Cleavage \[\to \] Gastrula \[\to \] Blastula
done
clear
C)
Cleavage \[\to \] Zygotes \[\to \] Blastula \[\to \] Gastrula
done
clear
D)
Zygote \[\to \] Blastula \[\to \] Cleavage \[\to \] Gastrula
done
clear
View Answer play_arrow
question_answer 141) Air quality indicator is :
A)
lichen
done
clear
B)
moss
done
clear
C)
algae
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 142) Green revolution is related with :
A)
Norman Borlaug
done
clear
B)
Sunder Lal Bahuguna
done
clear
C)
Reiter
done
clear
D)
Haeckel
done
clear
View Answer play_arrow
question_answer 143) Sea plants are an example of :
A)
xerophyte
done
clear
B)
mesophyte
done
clear
C)
hydrophyte
done
clear
D)
submerged plant
done
clear
View Answer play_arrow
question_answer 144) Pyramids of biomass in pond ecosystem is :
A)
inverted
done
clear
B)
upright
done
clear
C)
linear
done
clear
D)
irregular
done
clear
View Answer play_arrow
question_answer 145) Which of the following is false for Bt transgenic plant?
A)
Disease resistance
done
clear
B)
Prepared by Bacillus thuringiensis
done
clear
C)
It is recombinant type
done
clear
D)
No such plant is known
done
clear
View Answer play_arrow
question_answer 146) Reserpine is obtained from :
A)
Rauwolffia serpentine
done
clear
B)
coffee plant
done
clear
C)
tea plant
done
clear
D)
pea plant
done
clear
View Answer play_arrow
question_answer 147) Which of the following pair is correct?
A)
E.coli-E. histolytica
done
clear
B)
Culex-Elephantiasis
done
clear
C)
Bed bug-Kala-azar
done
clear
D)
Plasmodium-Sleeping sickness
done
clear
View Answer play_arrow
question_answer 148) Liver cirrhosis is caused by :
A)
excess alcoholism
done
clear
B)
no alcohol
done
clear
C)
due to bacterial infection
done
clear
D)
due to viral infection
done
clear
View Answer play_arrow
question_answer 149) Allergy involves :
A)
IgE
done
clear
B)
IgG
done
clear
C)
IgA
done
clear
D)
IgM
done
clear
View Answer play_arrow
question_answer 150) In recombination, vector used is :
A)
plasmid
done
clear
B)
E.coli
done
clear
C)
nucleic acid
done
clear
D)
cellulose
done
clear
View Answer play_arrow